Neuron Culture Medium
The product is a serum-free medium designed for primary culture of rat and murine neurons, and is suited for culturing cells of the central nervous system. The product contains culture supernatant of rat glial cells.
Features
Rapid production of mature neurons
- Approximately 1/2 of conventional culture method
- Neuron culture medium: 14 days
- Conventional culture medium: approximately 1 month
Low density culture
- 1/5 - 1/10 the number of cells compared with conventional methods
- Neuron culture medium: 0.1 ×106 cells/mL
- Conventional culture medium: 0.5~1.0×106 cells/mL
Ready-to-Use
- Cells can be cultured with the culture medium alone, with no need for additional supplements.
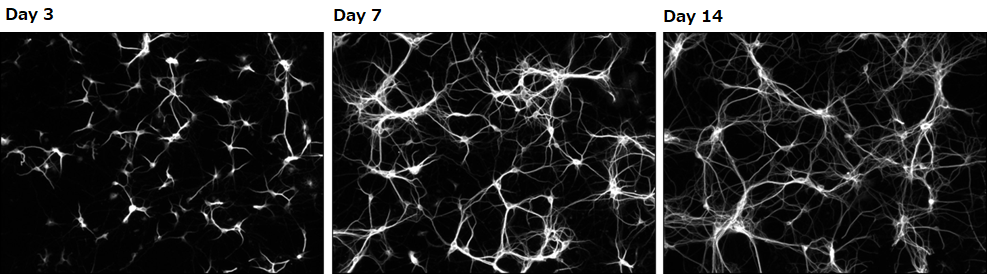
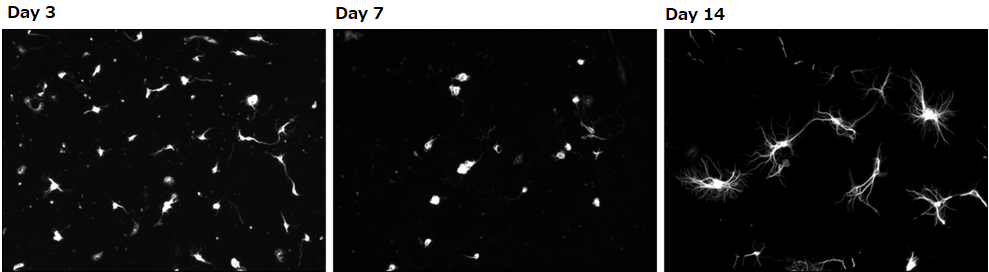
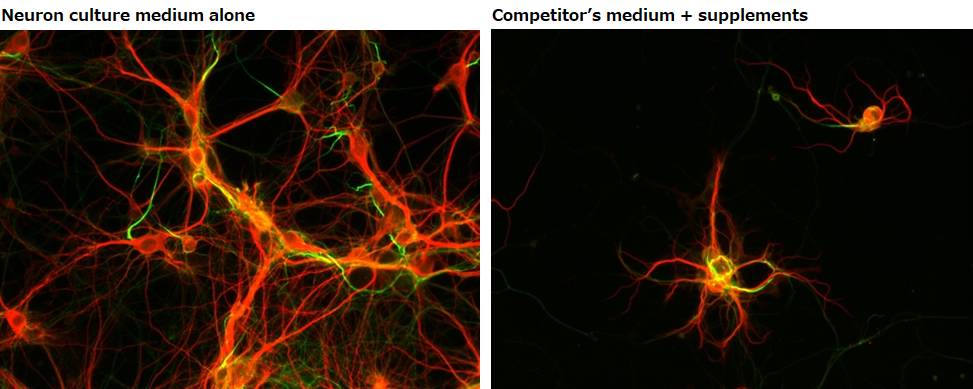
Confirming dendrite outgrowth
MAP2 immunostaining
Neuron culture medium alone
Competitor’s medium + supplements
Experimental conditions
Number of cells: 0.1×106 cells/mL(isolated from the hippocampus of a mouse at embryonic days 18.5)
Seeding density: 500 μL/well(polylysine-coated glass bottom dish)
Culture condition: Change half of the culture medium at days 3 and 7 (without the addition of Ara-C)
Data source
Dr. Hirotaka James Okano and Mr. Yuki Ogawa, Division of Regenerative Medicine, Jikei University School of Medicine
▶The data suggested that neurons grown in our culture medium had a faster rate of dendrite outgrowth compared with neurons cultured in the competitor’s medium.
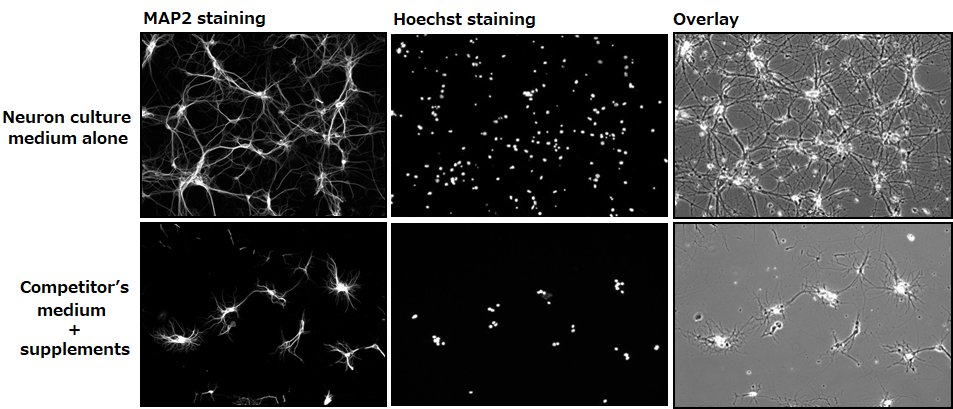
Confirming dendrite outgrowth and cell viability
MAP2 and Hoechst immunostainings
Day 14
Experimental conditions
Number of cells: 0.1×106 cells/mL(isolated from the hippocampus of a mouse at embryonic days 18.5)
Seeding density: 500 μL/well (polylysine-coated glass bottom dish)
Culture condition: Change half of the culture medium at days 3 and 7 (without the addition of Ara-C)
Data source
Dr. Hirotaka James Okano and Mr. Yuki Ogawa, Division of Regenerative Medicine, Jikei University School of Medicine
▶The data suggested that neurons grown in our culture medium had a faster rate of dendrite outgrowth and were more viable compared with neurons cultured in the competitor’s medium.
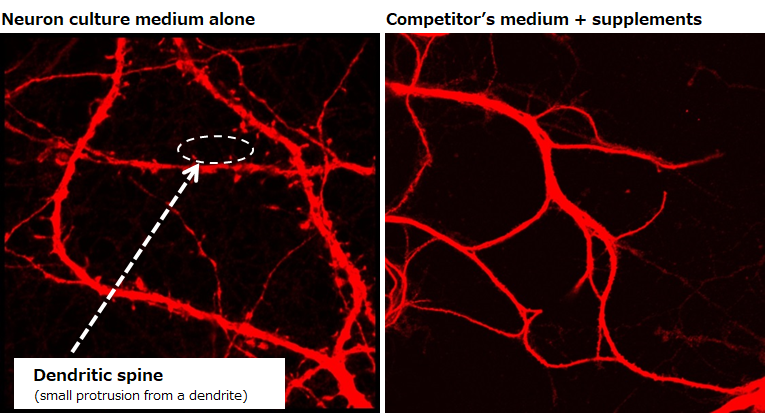
Evaluating the maturity of neurons
Examination of dendritic spines
Day 14
Experimental conditions
Number of cells: 0.1×106 cells/mL (isolated from the hippocampus of a mouse at embryonic days 18.5)
Seeding density: 500 μL/well (polylysine-coated glass bottom dish)
Culture condition: Change half of the culture medium at days 3 and 7 (without the addition of Ara-C)
Data source
Dr. Hirotaka James Okano and Mr. Yuki Ogawa, Division of Regenerative Medicine, Jikei University School of Medicine
▶The data revealed the presence of dendritic spines, which indicate maturity of neurons, on neurons cultured in our medium on day 14.
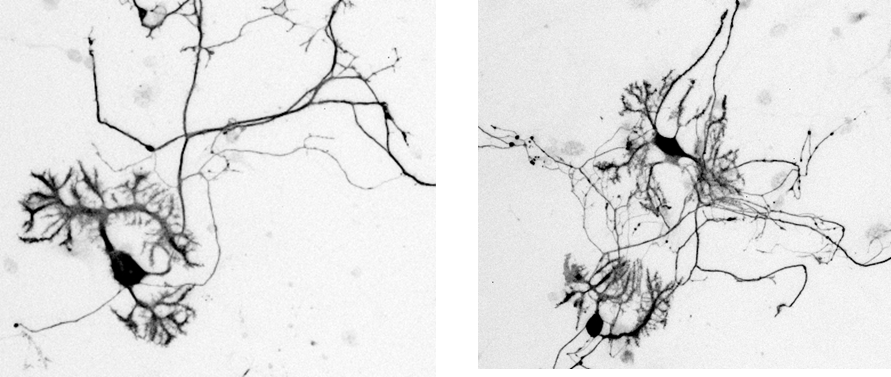
Confirming the maturity of neurons
Examination of dendrites and axons
Day 14
MAP2:Marker for dendrites
AnkyrinG:Marker for axon initial segment
Experimental conditions
Number of cells: 0.1×106 cells/mL(isolated from the hippocampus of a mouse at embryonic days 18.5)
Seeding density: 500 μL/well (polylysine-coated glass bottom dish)
Culture condition: Change half of the culture medium at days 3 and 7 (without the addition of Ara-C)
Data source
Dr. Hirotaka James Okano and Mr. Yuki Ogawa, Division of Regenerative Medicine, Jikei University School of Medicine
▶The data demonstrated that neurons cultured in our medium had multiple dendrites and one axon that are extended from the cell body, suggesting that they have properly matured.
Culturing murine Purkinje cells
Calbindin immunostaining
Day 14
Experimental conditions
Number of cells: 0.1×106 cells/well(isolated from the hippocampus of a mouse at embryonic days 18.5)
Seeding density: 500 μL/well (polylysine-coated glass bottom dish)
Culture condition: Final concentration at 1nM, supplemented with triiodothyronine
Data source
Dr. Hirotaka James Okano and Mr. Yuki Ogawa, Division of Regenerative Medicine, Jikei University School of Medicine
▶Purkinje cells cultured in 1 nM of our culture medium supplemented with triiodothyronine stained positive for calbindin and had dendritic development.
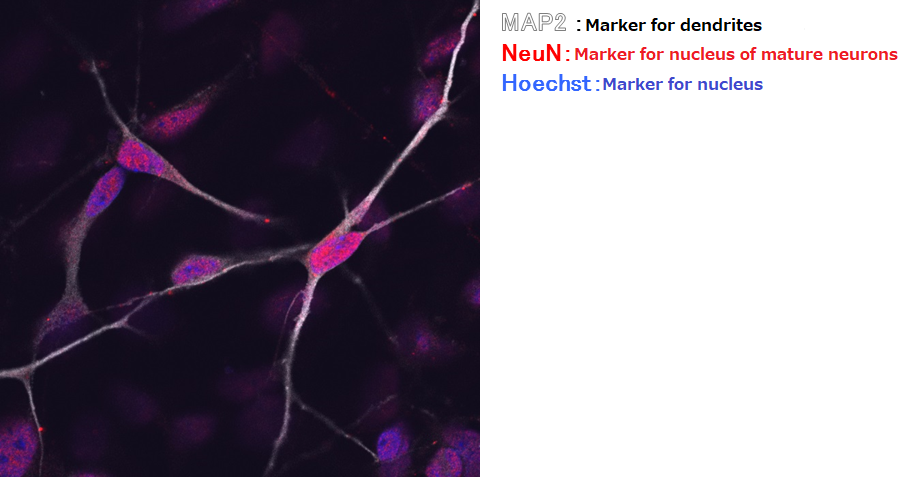
Confirming the maturity of human iPS-derived neurons
Immunostaining with MAP2, NeuN, and Hoechst
Suspension culture of cells after directed dopaminergic neuron differentiation at day 14 (42 days from directed differentiation)
Data source
Dr. Hirotaka James Okano and Ms. Keiko Bouno, Division of Regenerative Medicine, Jikei University School of Medicine
▶The data demonstrated that iPS-derived neurons cultured in our medium stained positive for NeuN, suggesting that they are mature neurons.
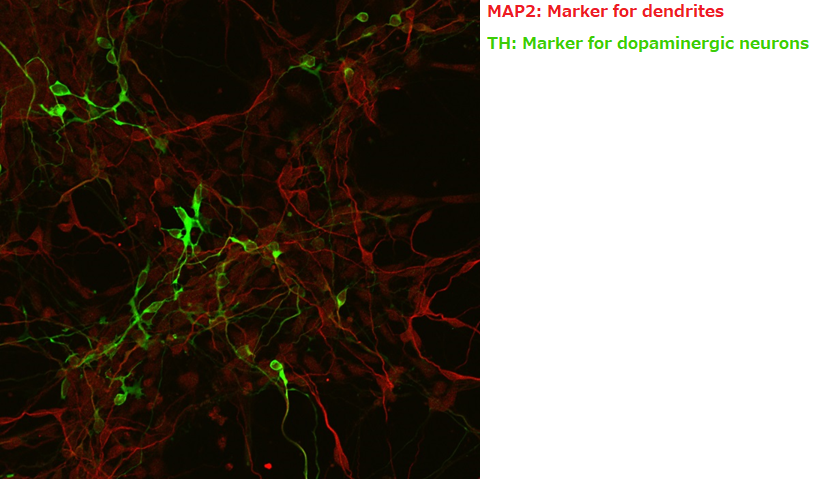
Immunostaining with MAP2 and TH
Suspension culture of cells after directed dopaminergic neuron differentiation at day 14 (42 days from directed differentiation)
Data source
Dr. Hirotaka James Okano and Ms. Keiko Bouno, Division of Regenerative Medicine, Jikei University School of Medicine
▶The data demonstrated improved viability of TH-positive cells derived from directed dopaminergic neuron differentiation when maintained in our culture medium.
Examining the physiological function of human iPS-derived neurons
Ca2+ imaging
Suspension culture of cells after directed dopaminergic neuron differentiation at day 14 (42 days from directed differentiation)
Endogenous fluorescence
Human iPS-derived neurons
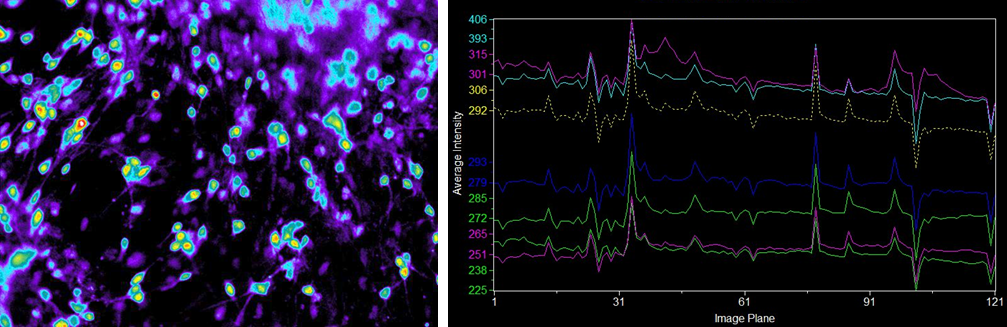
Fluorescence with the addition of ionomycin
Human iPS-derived neurons
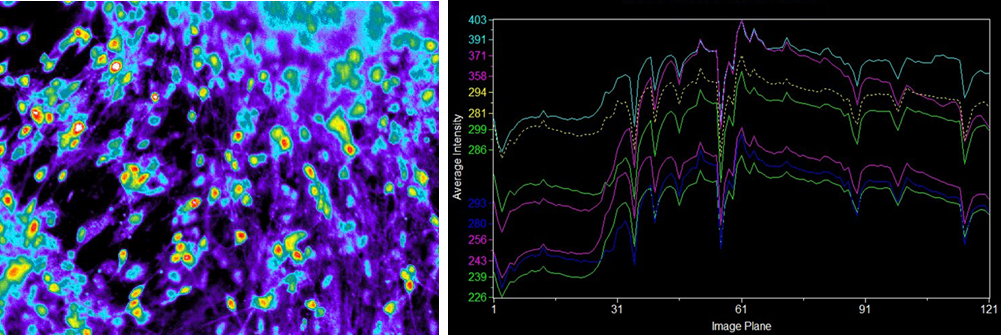
Data source
Dr. Hirotaka James Okano and Ms. Keiko Bouno, Division of Regenerative Medicine, Jikei University School of Medicine
▶Ca2+ imaging revealed that some of the human iPS-derived neurons cultured in our medium had independent physiological functions.
Whitepaper: Facilitating the maturation of iCell® GlutaNeurons
Product List
- Open All
- Close All
- Storage Condition :
- Keep at -80 degrees C.
Comparison | Product Number | Package Size | Price | Availability | Purchase |
|---|---|---|---|---|---|
| 100mL |
| In stock in Japan |
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.




