Pharmaceutical Raw Materials
Our pharmaceutical raw materials can be used in the manufacturing process of pharmaceuticals. The CertiPro series products, which are raw materials for pharmaceutical manufacturing, are classified into two product categories based on a quality management system; CertiPro (GMP compliant) and CertiPro-L (ISO9001 compliant).
For more information on these products, please see the dedicated website for pharmaceutical raw materials.
Product Line-up
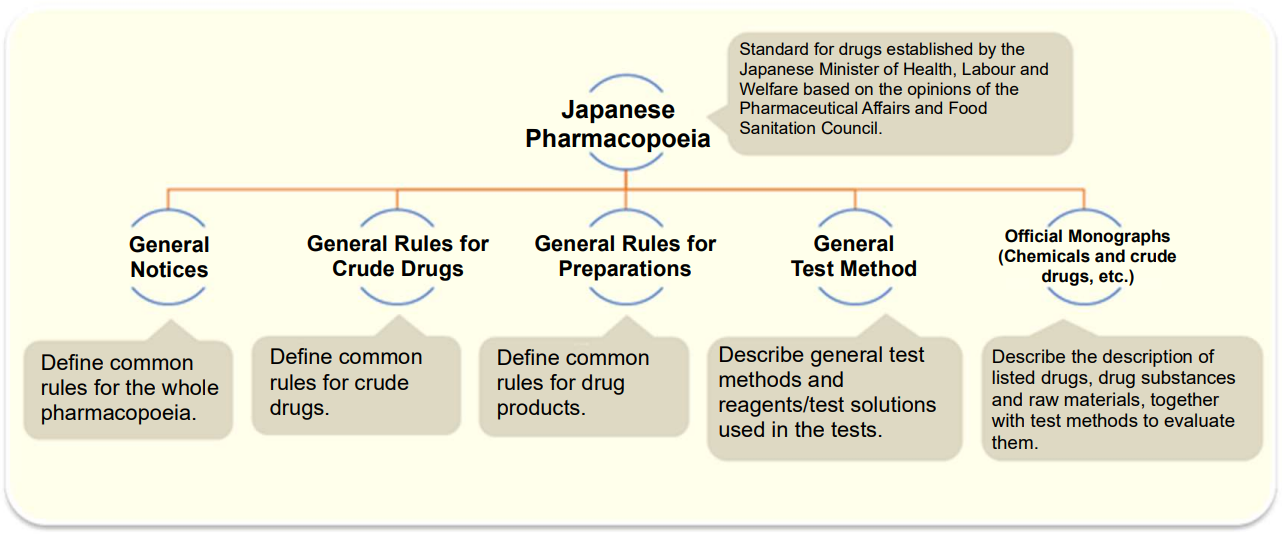
Japanese Pharmacopoeia (JP)
The Japanese Pharmacopoeia is a standard for drugs established by the Japanese Minister of Health, Labour and Welfare based on the opinions of the Pharmaceutical Affairs and Food Sanitation Council with the aim of ensuring appropriate description and quality of drugs, which mainly lists the drugs frequently used in Japan. It provides public standards required to ensure the quality of drugs in Japan and has been repeatedly revised in response to the progress of science and technology as well as international harmonization.
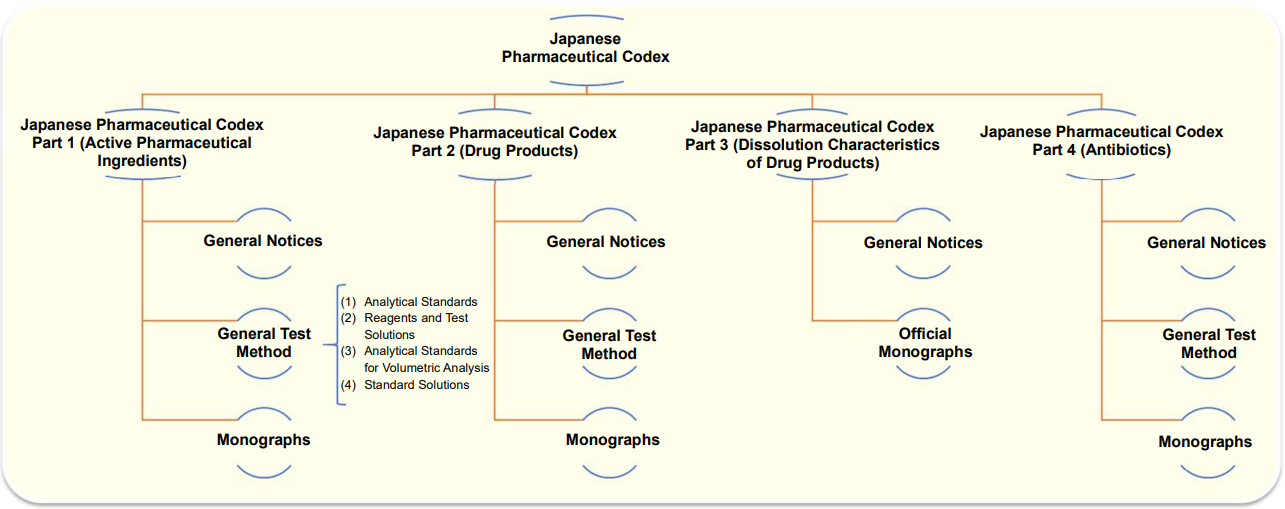
Japanese Pharmaceutical Codex (JPC)
The Japanese Pharmaceutical Codex is a standard for important ingredients that are not listed in the Japanese Pharmacopoeia.
It consists of Part 1 (active pharmaceutical ingredients), Part 2 (drug products), Part 3 (dissolution characteristics of drug products) and Part 4 (antibiotics), and describes general test methods as well as the description of listed drugs together with test methods to evaluate them.
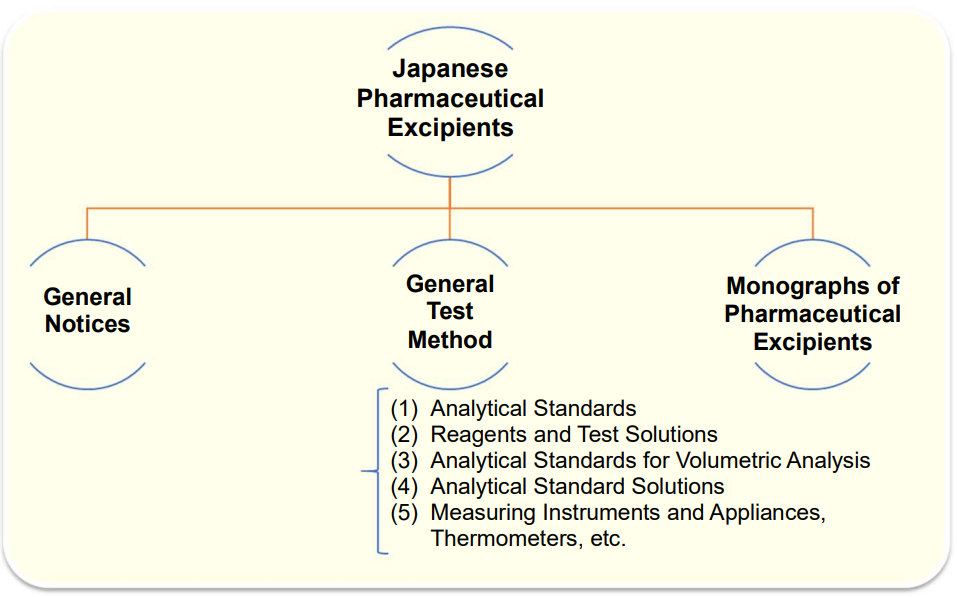
Japanese Pharmaceutical Excipients (JPE)
The Japanese Pharmaceutical Excipients provides a summary of specifications of pharmaceutical excipients that are not listed in the Japanese Pharmacopoeia.
It consists of General Notices, General Test Method?, and Monographs of Pharmaceutical Excipients, similarly to the Japanese Pharmacopoeia and the Japanese Pharmaceutical Codex.
Product Line-up
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.




