Dioxin & PCB Analysis
Dioxins have been considered highly toxic and cause reproductive and developmental problems. Since the amount of dioxin in the environment is extremely small, it is imperative to secure accurate and sensitive detection methods for qualitative and quantitative measurements. With this in mind, FUJIIFILM Wako has a large selection of reference standards, standard solutions, and mixed standard solutions used for the analysis of dioxins and PCBs (polychlorinated biphenyls).
FUJIFILM Wako Pure Chemical Corporation provides a wide variety of mixed standards.
More Information
An Overview on Methods for Analysis of Dioxins
Methods for the analysis of dioxins have been developed to be equivalent to international standards, so each nation has laid down these methods. However, as the demand for dioxins monitoring has increased, simple methods have been developed that can be performed quickly and at low cost. In some nations, such as the EU, the U.S. and Japan, have laid down simple methods for the analysis of dioxins. This article will explain methods for the analysis of dioxins, focusing on China’s trends.
Definition of dioxins
Dioxins are defined as the following three groups of organochlorine compounds;
- Polychlorinated dibenzo-para-dioxin (PCDDs)
- Polychlorinated dibenzofurans (PCDFs)
- Dioxin-like PCBs (Dl-PCBs)
Dl-PCBs possess similar toxicity to those of PCDDs and PCDFs. Each group includes their homologue and isomer with chlorine substituents in eight positions.
*Dl-PCBs could be called coplanar polychlorinated biphenyl (Co-PCBs).
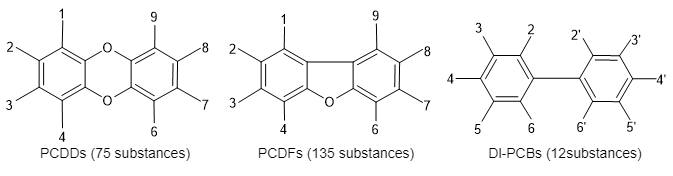
Figure 1 Dioxins
The United Nations Environment Programme(UNEP) provides the catalogue of all kinds of dioxins in the “Toolkit for Identification and Quantification of Releases of Dioxins, Furans and Other Unintentional POPs”, based on the ”Article of the Stockholm Convention”. In the Article 7 of the convention, each party are required to develop and submit to the conference of the parties a national implementation plan to fulfill their conditional obligations and to revise it as new substances are added. In addition, Article 5 stipulates that an action plan for reducing or eliminating unintentionally generated POPs such as PCDD and PCDF should be developed and implemented as a part of the domestic implementation plan.
Dioxins are not substances manufactured as industrial products, but substances generated by natural phenomena such as waste incinerators and forest fires. Dioxins are generated from exhaust gas and wastewater emitted from incinerators, herbicides and pesticides used on farms, and polychlorinated biphenyls (PCBs) used as insulating oil for electrical equipment.
Since the dioxins generation mechanism by combustion is complicated, the generation process has not been completely elucidated. It is said that dioxins are generated when precursors produced by burning organic compounds at low temperatures undergo a complex chemical reaction.
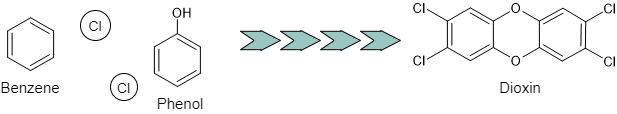
Figure 2 The mechanism of dioxins generation
Dioxin analysis
Typical analytical methods for dioxins include conventional instrumental methods, GC/HRMS, and biological assay.
Table 2 Applicability of dioxin analysis method to sample objects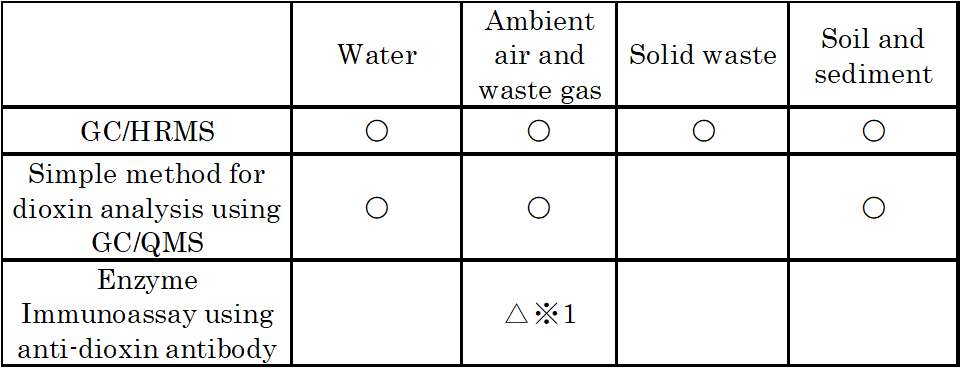
※1 applicable for soot and dust samples
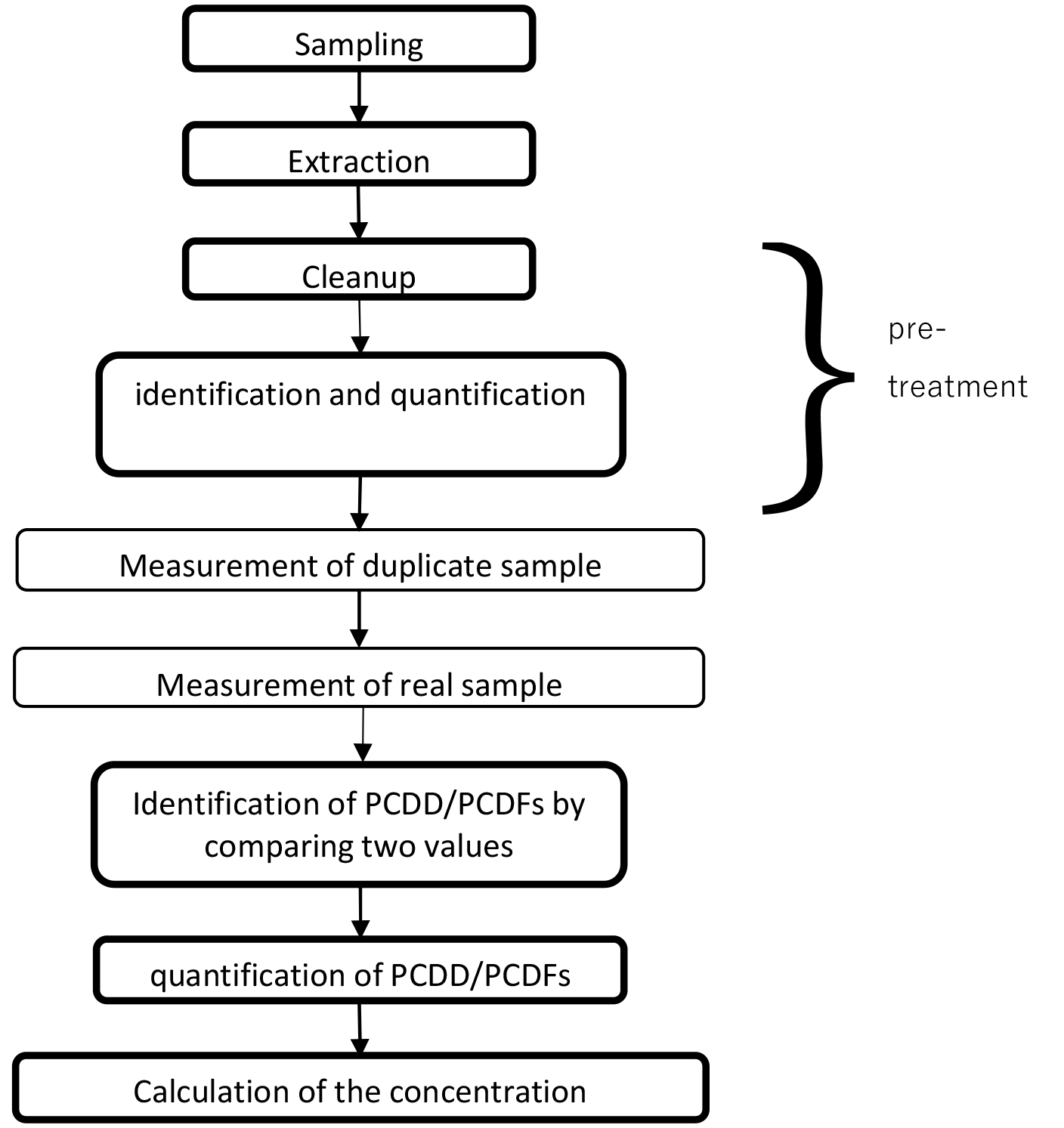
Figure 3 Steps in a typical dioxin analysis
The procedure for measuring dioxins is shown in figure 3. A sample is collected for each target, PCDDs and PCDFs are extracted, cleanup of them using silica gel column chromatography and reagents, and the identification and quantification of them prescribed in each method.
Typical methods for the analysis of dioxins are described as follows.
1. Gas chromatography/high-resolution mass spectrometry (GC/HRMS)
GC/HRMS consists of gas chromatography (GC) that can separate the mixture into individual component and mass spectrometry (MS). GC separates component according to the difference in the moving speed of the vaporized sample through the column. In the official analytical method for dioxins, each isomer of dioxins is determined by the GC / HRMS method.
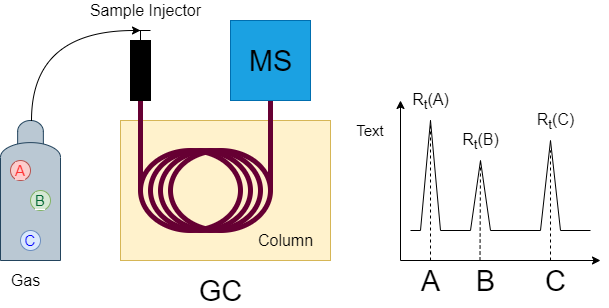
Figure 4 Dioxin analysis using GC/MS
Pretreatment reagents for dioxin analysis are listed as follows;
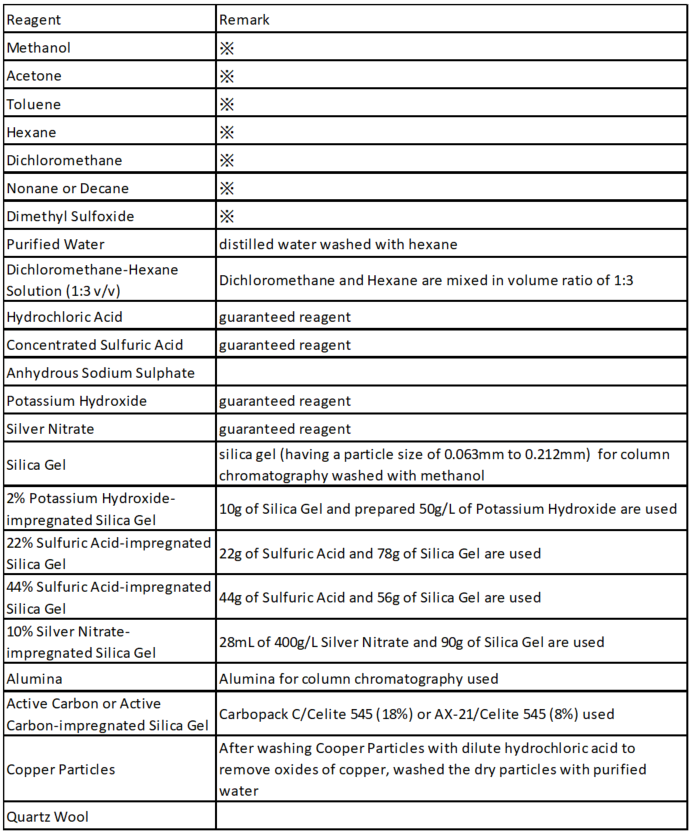
※Unless otherwise stated, reagents are required to have the quality of pesticide residue analysis. In the case of solvents, quality that guarantees that the content of dioxins is below the standard value is required by the test after concentration.
2. Quadrupole mass spectrometer (QMS)
GS is also used in simple methods for the analysis of dioxins as in the GC / HRMS method. However, low-resolution mass spectrometers such as quadrupole type and ion trap type are used. Quadrupole mass spectrometer (QMS) comprises four parallel cylindrical metal rods, which are charged by direct current. These rods scan sample ions based on their mass-to-charge ratio. This analysis method uses the same reagents as in GC/HRMS.
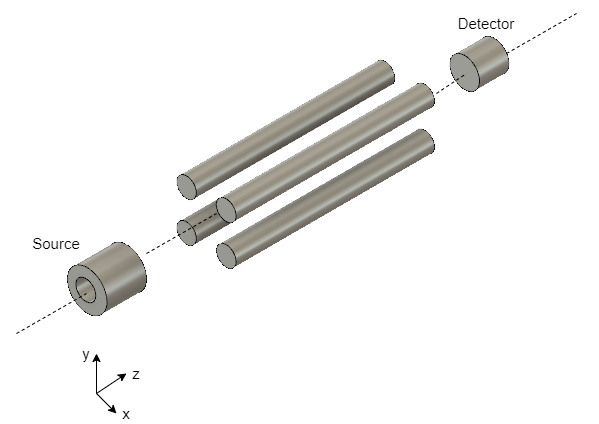
Figure 5 QMS
3. Bioassays
Bioassay is used to estimate the quantity, compounds, and efficacy of substances from the reaction of subjects, such as animals, plants, tissues, stimulated by them.
The pretreatment for bioassay is a method of extracting and cleaning up by multi-layer silica gel column treatment in the same way as the official method. There are two methods, using living tissues such as reporter gene assay and a toolkit composed by antigens reagents, such as enzyme immunoassay.
4. Enzyme Immunoassay (EIA) using anti-dioxins antibodies
Enzyme Immunoassay is used for an immunological measurement based on antigen-antibody reactions. This analysis method uses antibodies reacting specifically with dioxins.
References
- Samara, F., Gullett, B.K., Harrison, R. O., Chu, A,. Clark, G.C.:Environ. Int., 35, 588 (2009).
DOI: org/10.1016/j.envint.2008.11.003 - Batey, J.H.:Vacuum, 101, 410 (2014).
DOI: org/10.1016/j.vacuum.2013.05.005 - Fujimori, T., Kawamoto K.:J. Mater. Cycles Waste Manag., 30, 201 (2019).
DOI: org/10.3985/mcwmr.30.201
Related Pages
- Toolkit for Identification and Quantification of Releases of Dioxins, Furans and Other Unintentional POPs under Article 5 of the Stockholm Convention
- The text of the Stockholm Convention
- Information Brochure Dioxins 2009
- AGREEMENT ON TECHNICAL BARRIERS TO TRADE
- SW-846 Test Method 8290A: Polychlorinated Dibenzodioxins (PCDDs) and Polychlorinated Dibenzofurans (PCDFs) by High-Resolution Gas Chromatography/High Resolution Mass Spectrometry (HRGC/HRMS)
- SW-846 Test Method 4435: Screening For Dioxin-Like Chemical Activity In Soils And Sediments Using The Calux Bioassay And Toxic Equivalents (TEQs) Determinations
- Fundamentals of GC/MS
- Quadrupole Mass Analysers: An Introduction
- Standard Guidelines for the Environmental Monitoring of Chemicals
Product Line-up
- Dioxin Analysis Corresponding to Chinese Regulations
- DIOXIN TRAP BEADS
- Solvent for Dioxin Analysis
- SPE Column for Halogenated Dioxins Analysis
- 10% Silver Nitrate Impregnated Silica Gel
- SPE Column for Dioxin Analysis in accordance with JIS
- Silica Gel for Dioxin and PCBs Analysis
- Solvent for Residual Pesticide and PCB Analysis
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.