Fixatives
Autolysis and decomposition of living tissues begin immediately after excising. To observe the structure of cells/tissues and the localization of proteins and other molecules, it is important to fix the tissues and preserve them in a condition as close to that of the live tissues as possible for a long period of time. Fixation also minimizes the risk of deformation and deterioration of tissue specimens caused by chemicals, heat, and other factors during the subsequent processing. Fujifilm Wako offers a wide range of fixatives for light and electron microscopy.
More Information
Principles of Fixation
Many fixatives react with the functional groups of proteins, which are the major component of biological tissues, and form intra- and intermolecular cross-links. Fixatives significantly affect the morphology of the tissues and the structure and activity of target substances. Therefore, it is necessary to select the appropriate fixative according to the purpose.
Generally, formaldehyde is used in optical microscopy, and glutaraldehyde or osmic acid (osmium tetroxide) are used in electron microscopy. The principles of common fixatives are described below. Fujifilm Wako offers a wide range of fixatives for optical microscopy and fixatives for electron microscopy.
Formaldehyde
Formaldehyde first reacts with side-chain functional groups of proteins (amino, imino, amide, hydroxyl, carboxyl, SH, and aromatic groups) to form hydroxymethyl derivatives. The hydroxymethyl group further reacts with the active hydrogen of the protein to form an intra- or intermolecular methylene bridge (-CH2-). Proteins are immobilized and fixed in this process.
(1) R-H + CH2O → R-CH2(OH) (2) R-CH2(OH) + H-R' → R-CH2-R'
Formaldehyde preserves the activity of hydrolytic enzymes (alkaline phosphatases and esterases) but inhibits the activity of oxidoreductases (such as dehydrogenases) and transferases (phosphorylases). Although formaldehyde does not react with nucleic acids, lipids, or polysaccharides, these molecules are thought to be preserved when the surrounding proteins are cross-linked to form a gel.
Formalin
An aqueous solution of formaldehyde at approximately 37% (w/w) is called “formalin.” Fujifilm Wako's product “10% Formalin Solution” is a diluted 10% (w/w) formalin solution (formaldehyde content is 3.7-4.1%). Methanol is added to the formalin solution to prevent polymerization of formaldehyde.
Fujifilm Wako offers a line-up of 10%, 15%, and 20% formalin. Generally, a 10% formalin solution is used for fixation, but a higher concentration of formalin with high penetrating power may be used for faster fixation. However, this may cause damage to the specimen, and the choice should be made according to the purpose of the experiment.
Formalin is oxidized to formic acid when exposed to sunlight or air. Some tissues are affected by formic acid. To avoid the effect of formic acid, neutral-buffered formalin solution with added sodium phosphate is used.
Formalin has a pungent and unpleasant odor. Fujifilm Wako offers a product called Mildform, which contains a masking agent (wine extract) and has reduced formalin odor.
Glutaraldehyde
Glutaraldehyde also reacts with side chain functional groups of proteins (amino group, SH group, etc.), creating intra- or intermolecular cross-links.
Although glutaraldehyde does not penetrate the tissue as readily as formaldehyde and is unsuitable for the fixation of large specimens for optical microscopy, it is used for the fixation of specimens for electron microscopy because it preserves the morphology very well. However, lipids cannot be fixed, and the dehydration process results in the loss of membranous structure of the cell. To circumvent this issue, a two-step fixation with osmic acid is used for electron microscopy, as described below.
R-NH2 + R'-NH2 + CHO[CH2]3CHO → R-NH-CHOH[CH2]3CHOH-NH-R'
Osmic Acid (Osmium Tetroxide)
A 1% aqueous solution of osmium(VIII) oxide is called osmic acid. Osmic acid oxidizes ethylene groups of unsaturated lipids and amino, SH, and hydroxyl groups of proteins. The resulting peroxides form intra- or intermolecular cross-links (Figure 1).
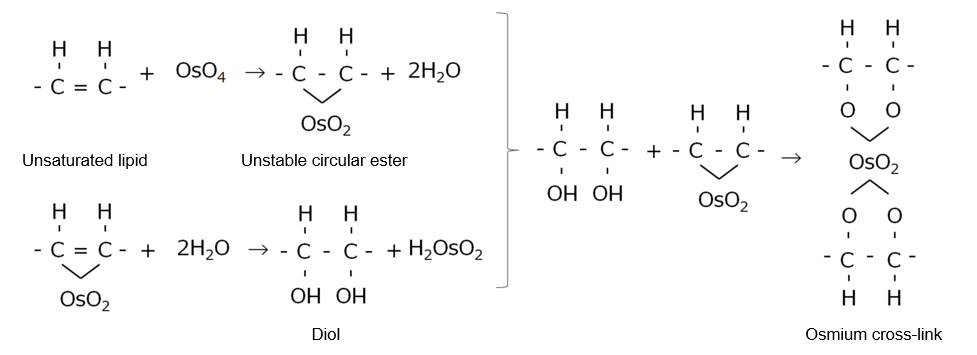
Figure 1. Reaction of osmic acid with unsaturated lipids
References
- Yamada, K.: “Histochemistry”, Nankodo, Japan, (1987). (Japanese)
- “Handbook for Staining and Bioimaging Experiments 5th ed.” ed. by Takata, K., Saito, N. and Kawakami, H., Yodosha, Japan, (2012). (Japanese)
- Okubo, K.: “Master of cell and tissue staining”, Yodosha, Japan, (2018). (Japanese)
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



