Lithium Ion Battery (LIB)
Lithium-ion batteries are used in a wide range of products around us because of their high energy density and voltage. Because of the development of electric vehicles and mobile devices, demand for high-capacity, high-voltage, and safe secondary batteries is growing. FUJIFILM Wako deal with various materials that constitute lithium-ion batteries. Our CLPA series of binders are polyacrylic acid (PAH) and its cross-linked polymer; these binders are synthesized by our technology and lead to significantly improved battery properties compared to those of polyvinylidene fluoride (PVdF). Furthermore, the addition of our WEA series of products [bis(sulfonate) type of materials] to an electrolyte of a secondary battery improves the battery properties.
More Information
Rough outline for mechanism of LIB system
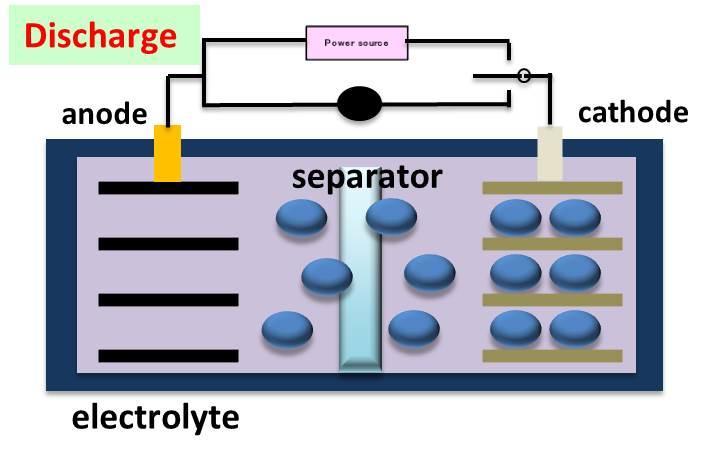
[Current]
Active material: Graphite
Theoretical capacity: 370 mAh/g
(Discharge) (Charge)
C6 + Li+ + e- ⇔ C6Li
[Next generation]
Active material: Silicon (Si)
Theoretical capacity: 4300 mAh/g
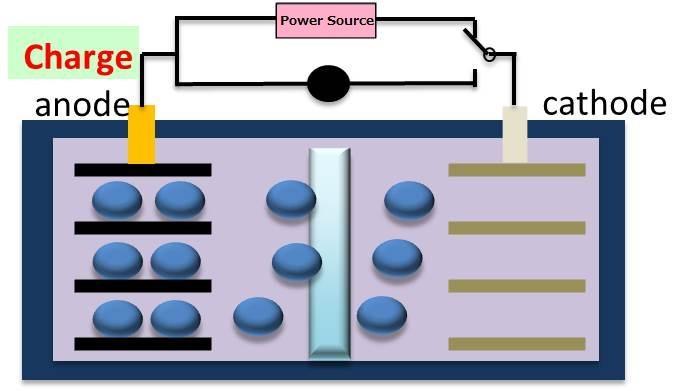
(Discharge ) (Charge)
Si + 4.4Li+ + 4.4e- ⇔ Li4.4Si
Si anode has potential more than decuple capability comparing with graphite anode.
Si anode technology is a highly anticipated development for LIB enhancement. Si anode technology will facilitate higher capacity and downsizing of the battery cell.
Difference between graphite and Silicon as anode
-
Graphite anode
Charge mode is intercalation system
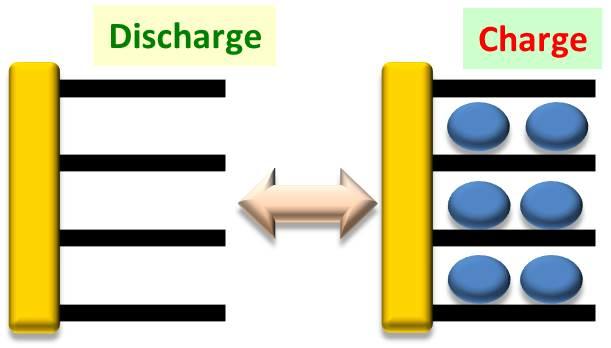
During charging, Li ions are inserted between layers of graphite. Currently, the graphite (anode) thickness does not change before/after a charging event.
WEA is good additive for SEI formation. -
Silicon anode
Charge mode is an alloy formation of Si-Li
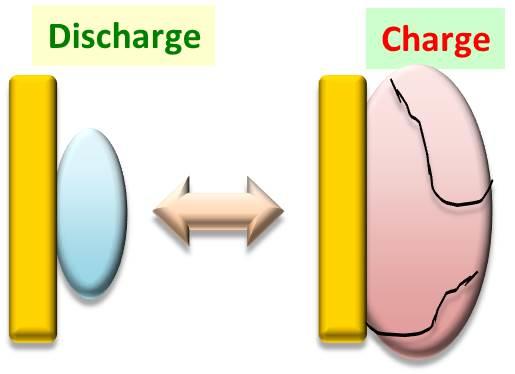
During charging, Li ions combine with Si to form an alloy of Si-Li, The Si-Li complex expands 4x under charge, while the Si anode shrinks to 1/4 its original size in the discharge state. Si anodes are damaged through the constant expansion and contraction.
The binder for Si is required a function to maintain the shape/size of Si anode.
FUJIFILM Wako develops CLPA in new binder for Si anode.
Additive for SEI formation
Relatively low LUMO (WEA-series)
→ formation of SEI with low electricity consumption
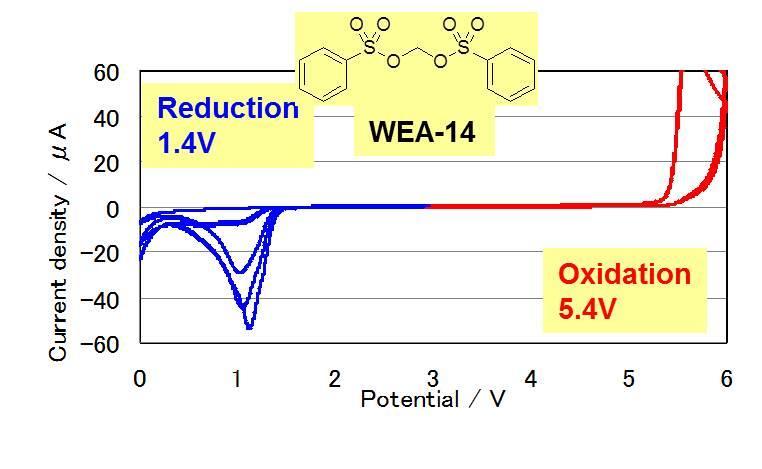
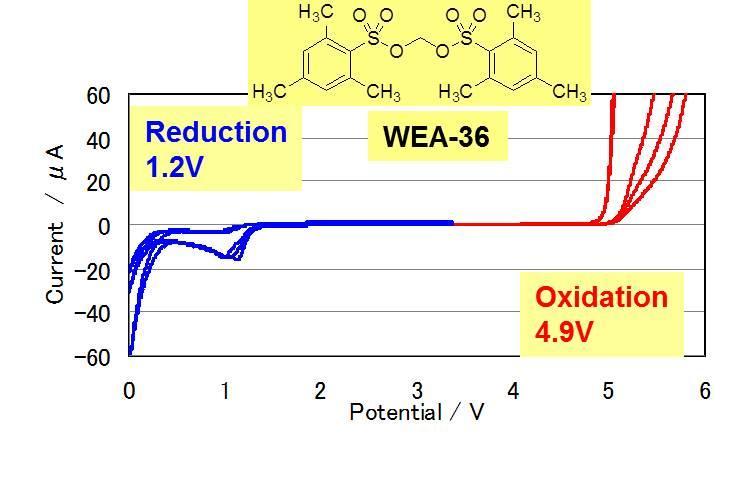
Cyclic Voltamograms of Electrolytes with Novel Additives (WEA)
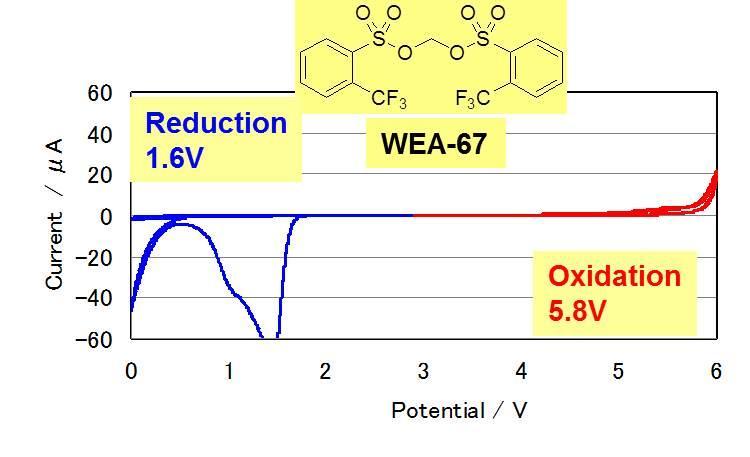
-
Reduction potential
WEA-67 > WEA-14 > WEA-36
(1.6V) (1.4V) (1.2V)LUMO energy
WEA-67 < WEA-14 < WEA-36
(1.44eV) (2.40eV) (2.50eV)
Performance of CLPA on Si and graphite composite electrode
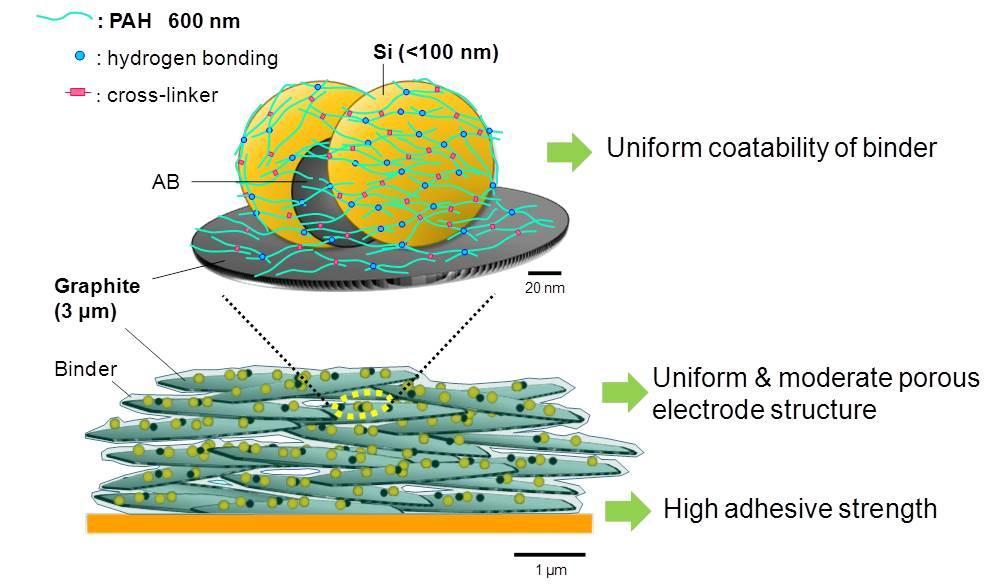
The detailed results are reported on following letter.
Reference
Komaba, S. et al.: Journal of The Electrochemical Society, 162 (12) A2245-A2249 (2015)
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.