Nucleic Acid Chemistry
"Nucleic acids" are a collective term for deoxyribonucleic acid (DNA) and ribonucleic acid (RNA).Nucleic acid synthesis has been actively studied, especially in the field of nucleic acid medicine.The traditional method for synthesizing oligonucleotides is called the phosphoramidite method, which is a form of solid-phase synthesis that involves the addition of a phosphoramidite monomer (monoamide of phosphite diester) to a solid-phase support.Our lineup of nucleic acid synthesis reagents focuses on ancillary reagents and dehydration solvents for use in the synthesis of oligonucleotides using the phosphoramidite method. We provide reagents of suitable quality for nucleic acid synthesis by leveraging our unique liquid preparation, synthesis, dehydration, and analysis technologies developed over years of reagent manufacturing.
Product Line-up
More Information
Nucleic acid drug
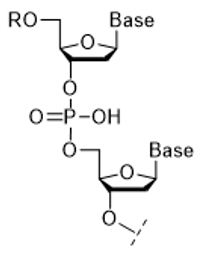
Fig 1. Oligonucleotide
Nucleic acid is a collective term for deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Oligonucleotides (Figure 1) are composed of bases, sugars, and phosphoric acid linked by phosphodiester bonds. Nucleic acid drugs," which have been the focus of much attention in recent years, utilize DNA and RNA molecules, which are the substance of genetic information of organisms, as pharmaceuticals, and are highly anticipated as next-generation pharmaceuticals because they can act on molecules in organisms that cannot be targeted by conventional small molecule drugs or antibody drugs1). Oligonucleotides, the main component of nucleic acid drugs, can be synthesized by organic synthesis (mainly solid-phase synthesis) using an automated synthesizing machine.
Types of Nucleic Acid
Drugs "Nucleic acid drugs" are drugs that target DNA or RNA in organisms and include antisense, siRNA, and aptamers. These nucleic acid drugs exert their medicinal effects by controlling the expression of target genes or inhibiting the expression of specific proteins.
Table 1: Major types of nucleic acid drugs
| antisense | siRNA | aptamer | decoy | CpG oligo | |
|---|---|---|---|---|---|
| Structure | single-stranded DNA/RNA |
double-stranded RNA |
single-stranded DNA/RNA |
double-stranded DNA |
single-stranded DNA |
| Base length | 12-30 | 20-25 | 26-45 | About 20 | About 20 |
| Target | mRNA Pre-mRNA | mRNA | extracellular protein | transcription factor | protein |
| Mechanism of action | mRNA degradation Splicing Control | mRNA degradation | dysfunction | transcriptional inhibition | Activation of innate immunity |
Phosphoramidite method
The traditional method for synthesizing oligonucleotides is called the phosphoramidite method, which is a form of solid-phase synthesis that involves the addition of a phosphoramidite monomer (monoamide of phosphite diester) to a solid-phase support. The fundamental reaction to bind the amidite, which is a nucleic acid monomer, consists of 4 steps:
Step 1 detritylation→ Step 2 amidite coupling reaction→ Step 3 capping reaction→ Step 4 oxidation or sulfurization. A cycle consisting of these 4 steps is repeated until the desired strand length is obtained (see Figure 1). An automated synthesizer is used for this reaction2).
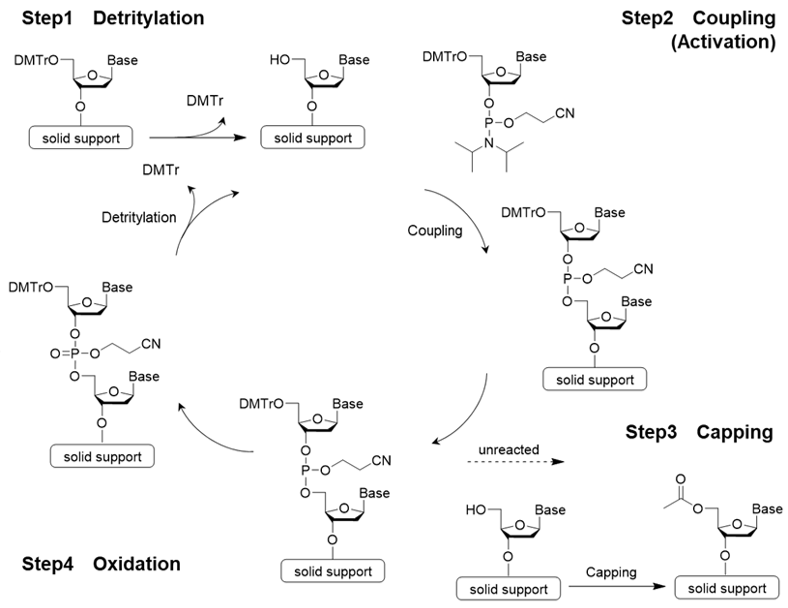
Fig 2. Reaction mechanism of phosphoramidite method
Reactions and reagents for phosphoramidite method3)
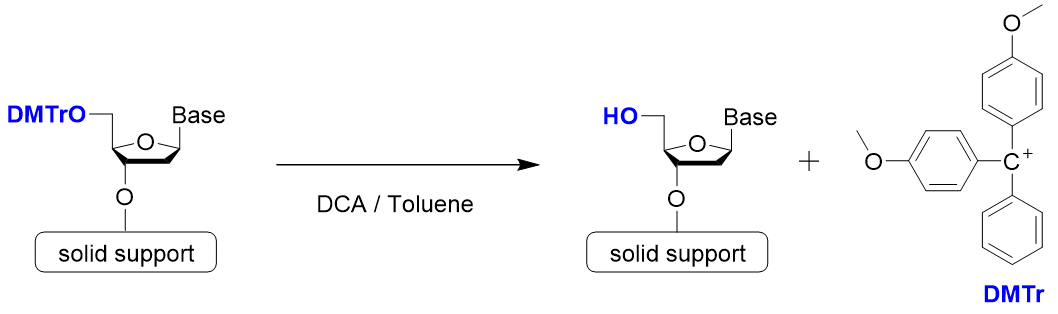
Step 1: Detritylation
Step 1 is detritylation. 4,4’-Dimethoxytrityl group (DMTr group) is often used to protect the hydroxy group at the 5’ position. Since the trityl cation is stabilized by two methoxy groups, it can be easily cleaved by treatment with a weak acid. Dichloroacetic acid or trichloroacetic acid is used as the acid.
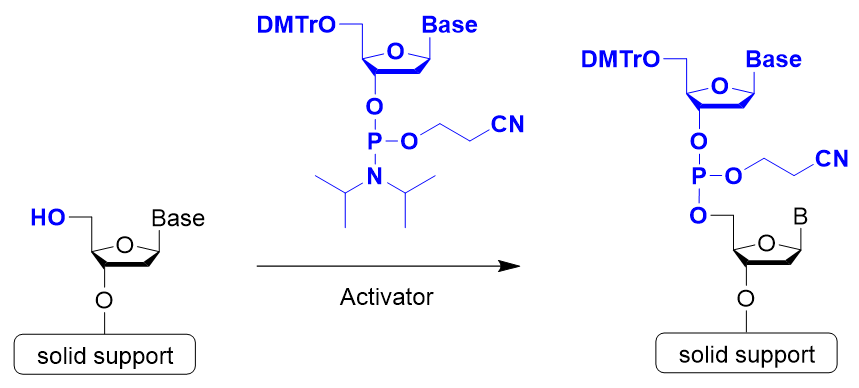
Step 2: Coupling Reaction (Activation)
Step 2 is the coupling reaction. Condensation occurs between the nucleotide unit to be bound and the nucleoside on the solid support or the oligonucleotide being synthesized. In general, 5-Benzylthio-1H-tetrazole (BTT), 5-Ethylthio-1H-tetrazole (ETT), or 4,5-Dicyanoimidazole (DCI) is used to activate phosphoramidite.
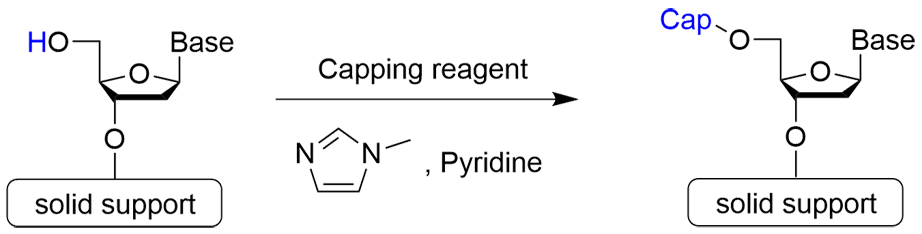
Step 3: Capping Reaction
Step 3 is the capping reaction. In the phosphoramidite method, an incomplete oligo that has one less base than the desired oligonucleotide may be formed if the coupling reaction does not progress completely and proceeds to the next step with an unreacted hydroxy group remaining. Therefore, the 5'-OH group of the unreacted strand is acetylated to prevent elongation reaction.
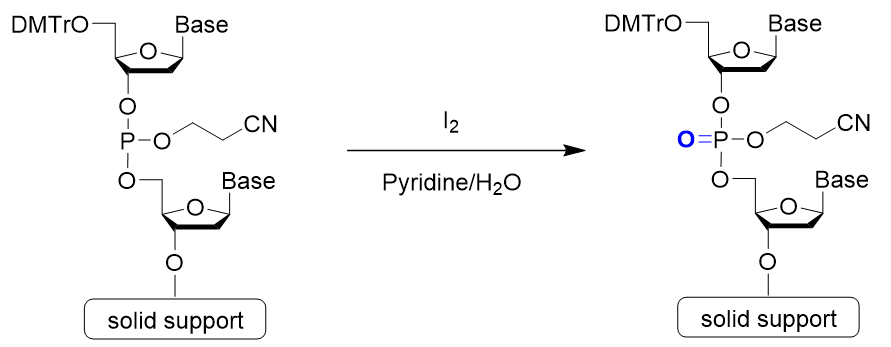
Step 4: Oxidation
Step 4 is oxidation. A phosphite ester bond is formed during phosphoramidite coupling. This is slightly unstable and may cause a side reaction during further elongation reactions. To prevent this, it is converted into a stable phosphate ester. Iodine (I2) is the most commonly used oxidizing reagent.
References
- [2016 Edition: Current status and future prospects of global nucleic acid drug development] 2016: Sekai no kakusaniyakuhinkaihatsu no genjyo to syoraitenbou (in Japanese). Seed Planning, Inc. (2016).
- "Development and Applications of Nucleic Acid Therapeutics" ed. by Takeshi Wada., CMC Publishing Co.,Ltd. (2016). (Japanese)
- [Biotechnology reagents] Baiotekunoroji shiyaku (in Japanese). The Chemical Daily Co., Ltd. (1989).
Product Line-up
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



