Tissue Clearing Reagents
Three-dimensional observation of the brain enable to understand complex networks of neural circuits. Traditionally, serial sections are imaged and reconstructed to observe the brain in three-dimensions; however, this method is problematic as it is laborious, and preparing and superimposing sections takes time and causes morphological damage to the tissue.
In this situation, tissue clearing technology is attracting attention as a new strategy for three-dimensional observation of the brain. Tissue clearing has been available for over 100 years, with constantly evolving performance, and recently has been used increasingly as a powerful means of observation of the brain. Here, we describes the principle of clearing and distinct uses, with a focus on clearing technology developed in Japan.
Product Line-up
More Information
Principle of Tissue Clearing
An important feature of tissue clearing is a reduction in light scattering. Usually, the refractive index differs between biological tissue and the solvent used (the liquid in which the biological tissue is immersed), and this difference can cause light scattering. Therefore, the tissue can be clarified by removing components with a high refractive index from the biological tissue and replacing the solvent with a liquid having a high refractive index to make the refractive index uniform.
Although some clearing techniques have been developed based on this principle, they pose problems, including fluorescent protein denaturation. For this reason, analyses that use these clearing techniques necessitate a combination of various staining methods. On the other hand, imaging-related technologies, such as microscopy and fluorescent proteins, have advanced remarkably, providing a technical basis for three-dimensional analysis of tissues. In such circumstances, there has recently been vigorous development of clearing techniques that are optimized to be combined with the latest fluorescent microscopy technology and fluorescent proteins enabling three-dimensional analysis of the deep tissue.
Tissue Clearing Technology
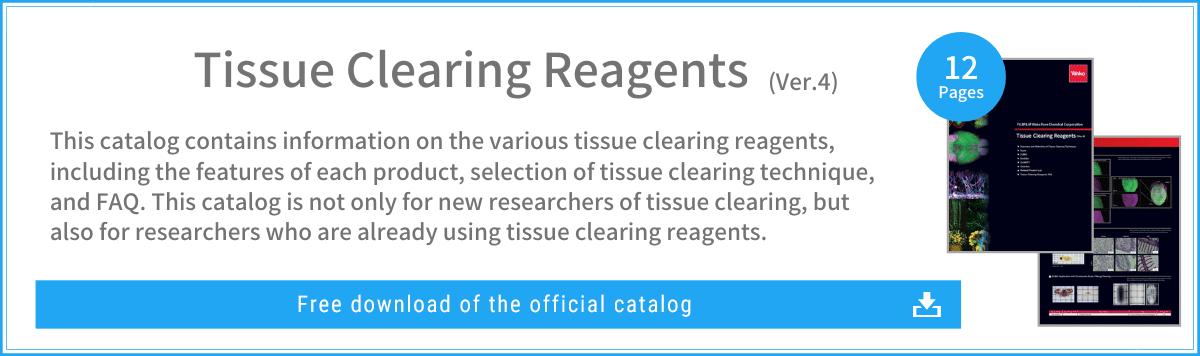
Scale (ScaleS, ScaleA2)
In conventional techniques for tissue clearing, organic solvents are commonly used but cause considerable loss of fluorescence intensity of fluorescent proteins, which require water molecules to emit light.
To solve this problem, the Scale method (a generic name for the ScaleS method and ScaleA2 method) was developed as a clearing technology based on an aqueous solution.1) 2)
With the Scale method, it is possible to clarify the tissue without loss of fluorescence intensity, using a mixed solution of urea, surfactant, and sorbitol (ScaleS method) or glycerin (ScaleA2 method) to suppress light scattering. Furthermore, the low concentration of surfactant used for defatting enables good retention of ultrafine structure, even after tissue clearing. As such, offering a good balance between transparency and structural retention, the Scale method is recommended for researchers who are trying to perform tissue clearing for the first time.
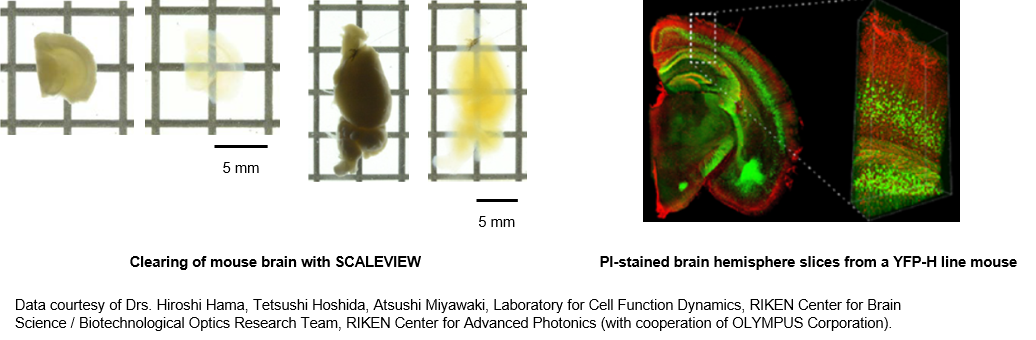
CUBIC
The CUBIC method is a tissue clearing technology that has been developed after re-examination of the three reagents used in the ScaleA2 method (urea, glycerin, surfactant).3) Transparency even greater than that obtained using the Scale method has been attained by replacing glycerin with amino alcohols. In addition, because the amino alcohols contained in the solution can remove heme, the major light absorbent in the tissue, this method is effective in the clarification of blood-containing organs.4)
There are two-types of the CUBIC method: 1st and 2nd. The 1st-generation CUBIC is recommended for beginners at tissue clarification because it allows the operator to visualize the clarification process and handle samples easily. The 2nd-generation CUBIC offers improved transparency compared with 1st-generation CUBIC.
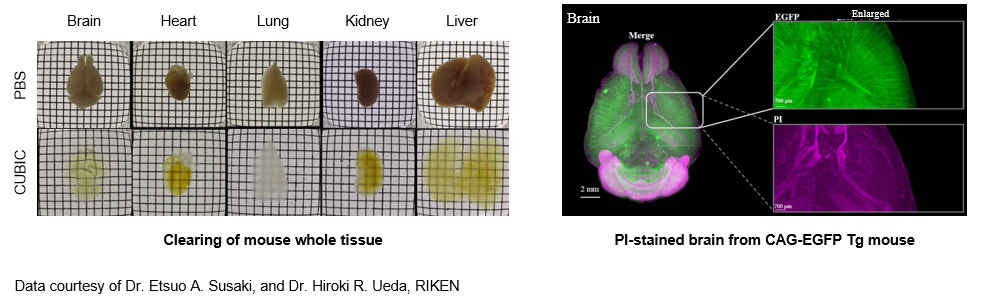
SeeDB2
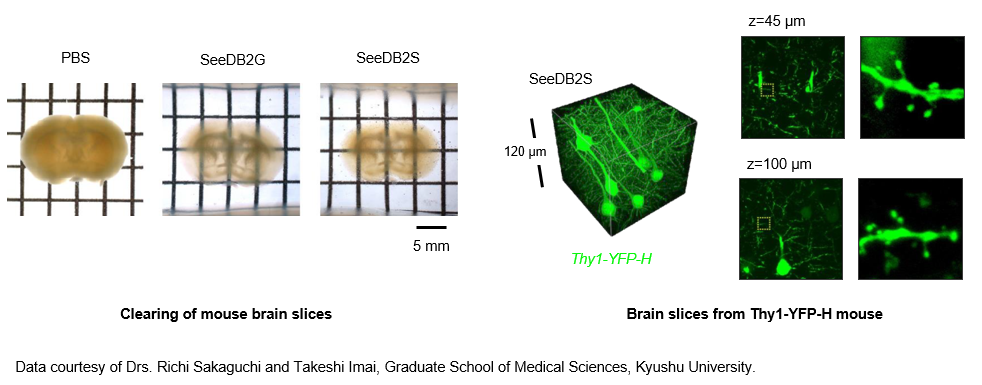
The CUBIC method and CLARITY (described later) method have been problematic, as transient changes in biological tissue size can affect fine morphology. The SeeDB2 method has enabled tissue clearing, while retaining ultrafine structure, by immersing the biological tissue in a saponin-containing iohexol base solution.5) The high refractive index of the solution used enables deeper imaging, minimizing the influence of differences in the refractive index. Although the transparency is lower than that seen with other techniques because surfactant is not used, extremely high performance for tissue structure retention is available, allowing morphological examination of dendritic spines by confocal microscopy or ultrahigh-resolution microscopy. Furthermore, the fluorescent protein is highly stable, so this method is suitable for the suppression of discoloration. On the other hand, dyes such as DAPI and Alexa are likely to cause loss of fluorescence intensity.
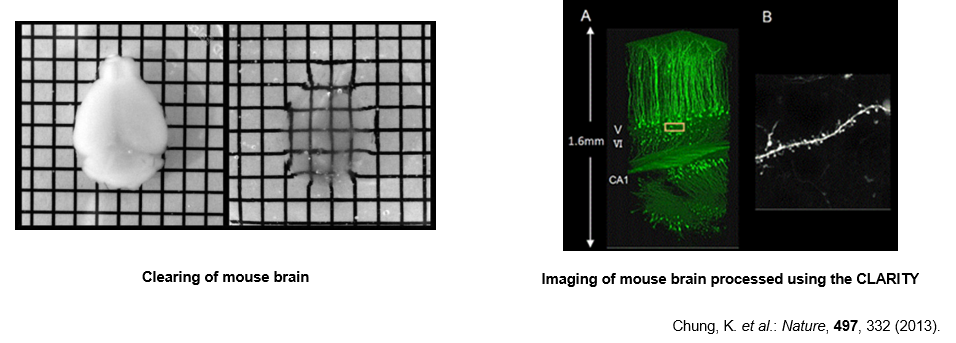
CLARITY
The most difficult task to perform during brain tissue clarification is elimination of lipids, which are abundant in the myelin sheath and elsewhere.
In the CLARITY method, lipids are eliminated by crosslinking and polymerizing brain proteins with polyacrylamide, and performing electrophoresis in an SDS-containing buffer.6) Although the operating procedures are more complicated than those of other techniques, taking approximately one week for clarification, substance permeability is high, making this technique suitable for antibody staining etc. because of active elimination of cell membrane components.
Selection of tissue clearing technique
Offering high reproducibility with simple procedures, the CUBIC method is best suited for whole brain imaging. Where problems arise due to sample brittleness and insufficient transparency, the CLARITY method is also effective. However, both are suitable for imaging at the cell or axon bundle level, rather than the microfine structure level, because the sample swells and the fine structure is not always retained.
For quantitative analyses of individual single axons, even down to dendrites (0.5 to several micrometers) and still finer structures (0.2 to 1 μm), such as dendritic spines, SeeDB2 is best, as it does not cause sample swelling or shrinkage. However, depths over 3 or 4 mm make it difficult to examine single axons.
The Scale method occupies an intermediate position between the CUBIC method and SeeDB2 method, in terms of clarification performance and tissue structure retention. Therefore, in cases where it is not necessary to examine fine structures using the SeeDB2 method, or when performing clarification on a trial basis, the Scale method is suitable. Organoids and spheroids can also be easily clarified using the Scale method.
Table 1. Features of tissue clarification technology
| Scale (ScaleS) | CUBIC | SeeDB2 | CLARITY | |
|---|---|---|---|---|
| Subject of observation | Whole brain Bone (decalcification required) Organoid/Spheroid |
Whole brain Whole tissue Bone (decalcification required) | Brain sections Organoid/Spheroid | Whole brain Whole tissue Bone (decalcification required) |
| Structure retention | High | Transiently swollen | Very high (no elongation or shrinkage) | Transiently swollen |
| Available dyes | Fluorescent protein Fluorescent dye | Fluorescent protein Fluorescent dye | Fluorescent protein Fluorescent dye (pay attention loss of fluorescence) | Fluorescent protein Fluorescent dye |
| Operational complexity | Very easy | Very easy | easy | Very complicated |
| Operating time | Approx. 1 day (2 mm-thick brain sections) | 1st-generation CUBIC: 10-15 days 2nd-generation CUBIC: 6 days (mouse whole brain) |
Approx. 3 days (2 mm-thick brain sections) | Approx. 1 week (2 mm-thick brain sections) |
References
- Hama, H. et al.: Nat. Neurosci., 14(11), 1481(2011).
Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain - Hama, H. et al.: Nat. Neurosci., 18(10), 1518(2015).
ScaleS: an optical clearing palette for biological imaging - Susaki, A. E. et al.: Cell, 157(3), 726(2014).
Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis - Tainaka, K. et al.: Cell, 159(4), 911(2014).
Whole-body imaging with single-cell resolution by tissue decolorization - Ke, M. T., et al.: Cell Rep., 14(11), 2718(2016).
Super-Resolution Mapping of Neuronal Circuitry With an Index-Optimized Clearing Agent - Chung, K. et al.: Nature, 497(7449), 332(2013).
Structural and molecular interrogation of intact biological systems - Imai, T.: Journal of Japanese Biochemical Society, 87(2), 225(2015).
3D fluorescence imaging using optical clearing agents (Japanese)
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.