Colloidal Titration
Colloidal titration utilizes the reaction between colloidal particles (a phenomenon in which polymer cations and polymer anions associate together). It is used in various fields as a simple quantitative method for polymer electrolytes.
At the endpoint of titration, an indicator (toluidine blue; TB) is added to react with the polyvinyl sulfate (PVS), and judgment is performed through the change of color from blue to red-purple.
Product Line-up
More Information
Principle of Colloidal Titration
The principle of colloidal titration is based on the fact that polycations (polymer cations) and polyanions (polymer anions) instantly form polyion complexes with their strong Coulomb attraction.
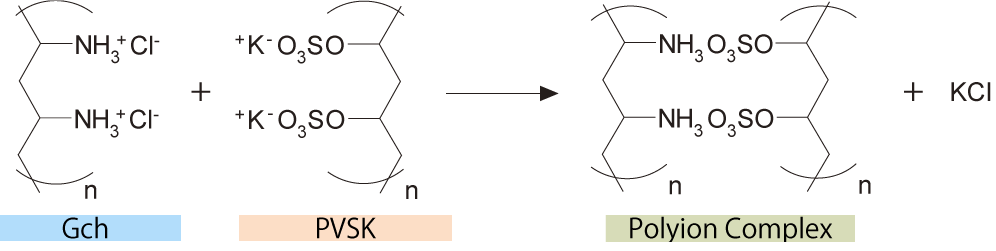
Titrate the N/400 solution of glycol chitosan (Gch) with the N/400 standard solution of potassium polyvinyl sulfate (PVSK). A white turbidity is produced gradually. At the endpoint of titration, toluidine blue (TB) is added as an indicator that changes color from blue to red-purple.
TB is a cationic dye that maintains the original blue color as long as the liquid is a cationic colloid. If PVSK becomes excessive at the endpoint of titration, it is adsorbed onto the anion colloid and changes the color to red-purple perceptively. The metachromasy phenomenon* (blue to red-purple) of an adsorptive indicator is used to detect the endpoint of titration.
*Metachromasy phenomenon: A phenomenon in which the stained tissues or cells stain differently from the original color of the dye when staining the cells or tissues.
Use of Colloidal Titrant
Standard Polyanion
Potassium polyvinyl sulfate (PVSK)
PVSK is a representative polymer acting as an anion colloid.
In FUJIFILM Wako, hexadecylpyridinium chloride (CPC), which has achieved SI traceability through the National Institute of Advanced Industrial Science and Technology (AIST), is used to add a factor of PVSK solution. This is a solution that can be used as a standard in colloidal titration.
Standard Polycation
Glycol chitosan (Gch)
Gch acts as a cation colloid only on the acidic side (pH 5 or less).
Methyl glycol chitosan (MeGch)
MeGCh acts as a cation in all areas of acid and alkali.
Poly (diallyl dimethyl ammonium chloride) (DADMAC)
Linear polymer, ideal polycation with uniform charge. Compared with Gch and MeGch, it is not affected by pH.
Precautions for Colloidal Titration
- pH
Colloidal titration utilizes the reaction between colloidal ions. It is necessary to react the positive and negative colloids with complete dissociation of each other. Gch can be used in acetic acid acidity (pH 5 or less), while MeGch can be used across the entire range of acids and alkalis. However, due to salt error, it is necessary to perform a blank test and determine the factor when used in strong acids or strong alkalis. - Temperature
As the metachromasy of toluidine blue becomes unclear at high temperatures, it is recommended to cool the analytical sample to room temperature as much as possible before performing the test. - Salt concentration
Attention should be paid to the salt concentration since it affects the dissociation of polymer electrolytes. In particular, Gch is greatly affected since polyvalent ions cause a reduction of factors.
Perform a blank test when titrating the samples with salt concentrations above 0.5%. If possible, dilute the sample or dialyze to remove the salt.
Application examples
- Food analysis: Assay of pectin, assay of tannin, assay of alginic acid, and assay of agar
- Civil engineering: Application to water treatment (cleaning of sludge water)
- Pulp engineering: Assay of lignin in wood
Reference
Sente, R., et al: “Colloid titration method” (NANKODO) (1969)
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



