Reagents for mRNA Synthesis Research
mRNA drugs and mRNA vaccines are types of mRNA preparations used to prompt the body to produce specific proteins for treating and preventing diseases. The application of mRNA drugs and vaccines is most advanced in the field of vaccine development, where the aim is to combat infectious diseases. However, they are also expected to be useful in treating various other conditions such as genetic disorders and cancer.
Fujifilm Wako supplies reagents for mRNA synthesis research including pseudouridine and 1-methylpseudouridine.
More Information
Challenges of mRNA Drugs and Vaccines, and RNA Modifications
mRNA drugs and vaccines are mRNA molecules that are designed to express target proteins or antigens in the body for the prevention or treatment of diseases. Like DNA-based gene therapy and DNA vaccines, mRNA drugs and vaccines involve expressing proteins that have preventive or therapeutic effects against diseases. The differences are that mRNA does not need to be transported to the cell nucleus, thus reducing the risk of insertion into the genome, and that the time from mRNA administration to protein expression is short because only translation takes place. These are the main advantages of mRNA drugs and vaccines. On the other hand, the challenges of mRNA drugs and vaccines include the fact that mRNA is unstable and easily degraded, as well as the immunogenicity of mRNA itself. For these reasons, bringing mRNA drugs into practical use has faced considerable hurdles.
When administered to the body, mRNA stimulates Toll-like receptors (TLRs) that recognize exogenous nucleic acids of bacterial or viral origin, thereby triggering an inflammatory response. One way to reduce the immunogenicity of mRNA is the use of modified nucleosides. In eukaryotes, modifications to ribose and some of the bases of RNA are known to occur, and more than 100 different RNA modifications have been reported to date. RNA modifications are thought to contribute to conformational stabilization and subcellular localization of RNA, though their roles are not fully understood.
Against this backdrop, in 2005, Karikó and colleagues discovered that TLR-mediated immune responses were reduced by replacing bases constituting mRNA with modified nucleosides such as 5-methylcytidine, N6-methyladenosine, and pseudouridine. In 2008, they reported that the translation efficiency was greatly improved with mRNAs containing pseudouridine. Various modified nucleosides have since been explored, and some of the mRNA vaccines against COVID-19 contain 1-methyl pseudouridine, which resulted in even higher translational efficiency than pseudouridine-containing mRNA.
Chemically synthesized pseudouridine and 1-methylpseudouridine are available from Fujifilm Wako.
mRNA Synthesis Method
An enzymatic method called in vitro transcription is commonly used for the synthesis of long RNAs such as mRNA drugs and vaccines. Linear double-stranded DNA is used as a template and transcribed into mRNA using nucleoside triphosphates, the building blocks of mRNA, and the enzyme RNA polymerase. RNA polymerase binds to the promoter region of the DNA and synthesizes mRNA by connecting complementary nucleoside triphosphates one after the other, using one strand of the DNA as a template, as it moves in the direction from the 5' end to the 3' end (Figure 1).
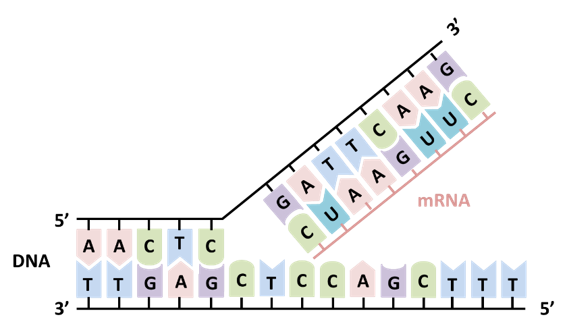
Since mRNA synthesized by in vitro transcription reactions does not have the cap structure, it is necessary to create one during the synthesis process. There are two ways to create the cap structure: by using a capping enzyme after transcription, or by using a cap analog such as anti-reverse cap analog (ARCA) during the transcription reaction. With the method using ARCA, both transcription and capping can be performed in one step, and the cap structure is easily created. The low capping efficiency, however, has been a challenge. To overcome this problem, new capping analogs with improved capping efficiency have been developed.
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



