FAQ
We introduce standard answers to frequently asked questions from customers on a daily basis on Q & A format.
Bacterial Endotoxin test
-
Q1.How to dissolve Standard Endotoxin?
-
A.Japanese Pharmacopoeia Standard Endotoxin (JP-RSE): Add endotoxin test water to make 10,000EU/mL, and stir it vigorously for 5 minutes.
US Pharmacopoeia Standard Endotoxin (USP - RSE): Add 5 mL of endotoxin test water (2,000EU/mL) and stir it vigorously for 30 minutes.
Control Standard Endotoxin (CSE): Add endotoxin test water to make 1,000EU/mL and stir it vigorously for 2 minutes.
-
Q2.When conducting a test in accordance with the Japanese Pharmacopoeia, which Standard Endotoxin should be used?
-
A.Be sure to use the pharmacopoeial Standard Endotoxin. (JP-RSE, USP-ESE, EP-RSE)
-
Q3.Tell me the proper use of Gel-clot technique, Turbidimetric technique and Chromogenic technique.
-
A.Please use Gel-clot technique to semi-quantify, and Turbidimetric technique or Chromogenic technique to quantify.
Basically there is no big difference between Turbidimetric technique and Chromogenic technique. However, there are differences in the measuring devices, the measuring range of reagents and the cost. Please select the measurement method after considering them.
If there is a doubt or dispute about the measurement result by Gel-clot technique, Turbidimetric technique and Chromogenic technique, please make a final judgment by the result of Gel-clot technique.
-
Q4.Is it necessary to use debicated tools?
-
A.It is not necessary to use debicated tools. However, endotoxin and β-glucan-free containers have to be used. Endotoxin can be inactivated in glass containers by dry heat sterilization at over 250 ℃ for more than 30 minutes. Since metal ions (Fe, Al, Ga, Cr etc) eluted in a trace amount may influence the test, please avoid using metal containers. Please use the disposable plastic containers etc except for those with manufacturer's guarantee, after confirming below.
- They have no contamination of endotoxin.
- They do not adsorb endotoxin.
- They have no eluted substance influencing the test result.
- They have no contamination of endotoxin.
-
Q5.How can the limit for bacterial endotoxins of parenteral drugs be determined?
-
A.
For the samples whose endotoxin standard values are not determined under each Article of Pharmacopoeia, please set as follows.
Endotoxin limit(EU/mL) = K value (EU/kg)/Human maximum total dose administered (mL/kg) * 1
*1: When injections are administered frequently or continuously, they should be the maximum amount to be administered within one hour.
[ Reference ]
K value defined in Japanese, the US and European Pharmacopoeia (threshold pyrogenic dose of endotoxin)Intended Route of administration K value (EU/kg) Intravenous 5.0 Intravenous for radiopharmaceuticals 2.5 Intraspinal 0.2
-
Q6.How should MVD (Maximum valid dilution) be calculated?
-
A.
Please apply to the following calculation formula, then confirm.
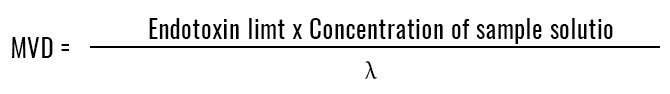
λ: ※ Labeled Gelation sensitivity by Gel-clot technique and the minimum concentration of standard curve by photometric method
Our Toxinometer and Toximaster QC which is the software attached to MPR Endotoxin Measurement System have the function to calculate MVD automatically.
-
Q7.How to preserve the samples?
-
A.
Please check the following matters and preserve them.
- No live bacteria grow in the sample
- The endotoxin value does not change depending on the storage period
- Containers used for storage should be confirmed to be free of endotoxin contamination, endotoxin adsorption and eluted substance influencing the test result.
- No live bacteria grow in the sample
-
Q8.Please tell me how to adjust pH of samples.
-
A.
If the pH of sample solution is not in the rang of 6.0 to 8.0, pH adjustment is necessary.
(The optimum pH of Bacterial Endotoxin test is around 7.3, but because the buffer component is contained in Lysate reagent in advance, it can be measured within pH6.0 - 8.0.)
If the sample can be diluted, the pH is adjusted by dilution. For samples that can not be diluted, pH adjustment is done by adding a dilution solution of NaOH or HCl to the sample solution.
Alternatively, it is possible to dilute the sample solution with a buffer solution. For pH adjustment, please use NaOH and HCl which are prepared beforehand.
-
Q9.Can the test tubes for measurement be reused?
-
A.Test tubes for measurement can not be reused. However, test tubes for dilution and sample storage can be reused by dry heat sterilization at 250℃ for at least 30 minutes.
Measurement devices
-
Q10.What is the difference between Endpoint Assay and Kinetic Method?
-
A.
-
Endpoint Assay
Method utilizing the dose-response relationship between the turbidity or the magnitude of absorbance and endotoxin concentration after a certain reaction time. -
Kinetic Method
Method utilizing the dose-response relationship between the time at which a constant turbidity or absorbance has been reached and endotoxin concentration, or that between the over-time change rate of turbidity or absorbance and endotoxin concentration.
-
Endpoint Assay
-
Q11.Please tell me the measurement principle of Toxinometer.
-
A.
Please refer to the following.
-
Q12.Can I change the threshold value, waiting time and count when using a Toxinometer?
-
A.It is possible to change them. However, the performance of reagent can not be guaranteed in that case.
-
Q13.Please tell me the measurement wavelength of Toxinometer® ET-7000.
-
A.The center wavelength is 430 nm.
Measurement reagents
-
Q14.What is an Endotoxin-specific Reagent?
-
A.
Normally, in a Lysate reagent, the reaction against (1 → 3)-β-D-Glucan of a cell wall constituent component of fungus also occurs in addition to endotoxin. Reagents prepared to react only with endotoxin are called Endotoxin-specific Reagents.
-
Q15.What is the difference between single test and multi-test?
-
A.
- Single test
A type that one dose of Lysate reagent is dispensed beforehand in a reaction tube -
Multi test
A type that is used by dissolving Lysate reagent in endotoxin test water and dispensing necessary quantity of it in a reaction tube or plate.
- Single test
-
Q16.Though the sample amount is different between single test and multi-test, is it necessary to think about the difference in concentration?
-
A.There is no problem because that reagents are prepared according to the amount of sample to be added.
However, since the amount of sample to be added is different, if any reaction interfering factor is included in the sample, the inhibition and promotion will be affected in a different way.
-
Q17.Please tell me the storage temperature of Lysate reagent.
-
A.
All Lysate reagents should be stored under refrigeration.
However, please save the dissolved Lysate reagent under the condition set for each reagent. When using it later, please cryopreserve it at the storage temperature
※ set for each Lysate reagent and use it within 2 weeks. Please do not repeat freezing and thawing more than one time.ES-Ⅱ Series: -10 ℃ to -30 ℃
ES-F Series, ES-F/Plate: -10 ℃ to -20 ℃
Color KY Series: -80 ℃ or less



