Recombinant Cascade Reagent (rCR) for Bacterial Endotoxin TestPYROSTAR™ Neo+
- Chromogenic Technique
- Endotoxin Specific
- Recombinant
Endotoxin, a major pyrogen, is commonly known as lipopolysaccharide (LPS) that is a component of the cell wall of Gram- negative bacteria. If parenteral drugs are contaminated with endotoxin, it may cause serious symptoms such as fever and shock. Bacterial Endotoxin Testing (BET) is performed using a Limulus Amebocyte Lysate (LAL) reagent made from horseshoe crab blood extracts. This is based on the phenomenon that horseshoe crab amebocyte coagulates in the presence of endotoxin. PYROSTAR™ Neo+ consists of recombinant Factor C, Factor B, and proclotting enzyme, as well as chromogenic synthetic substrate, and buffer components. Endotoxin can be measured by the chromogenic substrate method in the same way as the conventional LAL reagent.
Features
- ➢ Less variations in reactivity between reagent lots by utilizing recombinant proteins instead ofhorseshoe crab amebocyte.
- ➢ Equivalent reactivity and reproducibility to conventional LAL reagents.
- ➢ Endotoxin-specific [No Factor G is included which is activated by (1→3)-β-D-glucan and activate pro-clotting enzyme.]
- ➢ Colorimetric quantification with a microplate reader or Toxinometer™ .
Principle
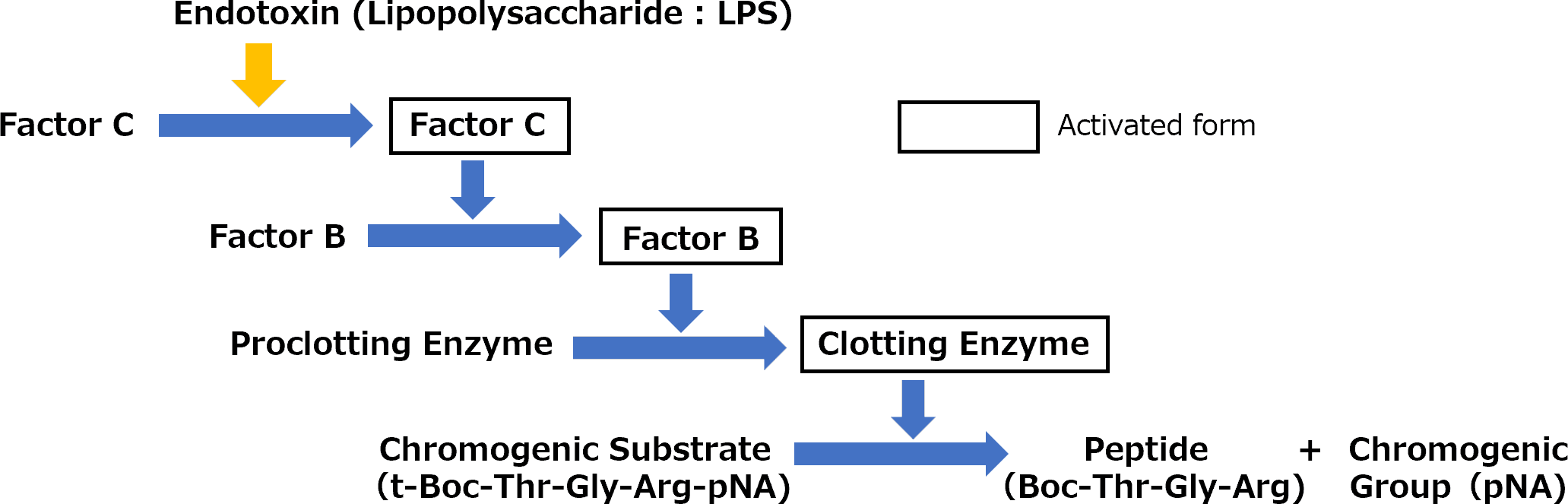
PYROSTAR™ Neo+ contains three recombinant proteins, horseshoe crab Factor C, Factor B, and proclotting enzyme, and chromogenic synthetic substrate t-Boc-Thr-Gly-Arg-pNA. When endotoxin is present in the sample to be tested, endotoxin activates Factor C and subsequent cascade reaction occurs as shown in the figure below, resulting in cleavage of the chromogenic substrate and release of para-nitroaniline (pNA). The amount of endotoxin in the sample is determined by the relationship between the standard endotoxin concentration and time which takes for the absorbance to reach a predetermined value, i.e., the time which takes for the amount of free pNA to reach a certain concentration.
How to use
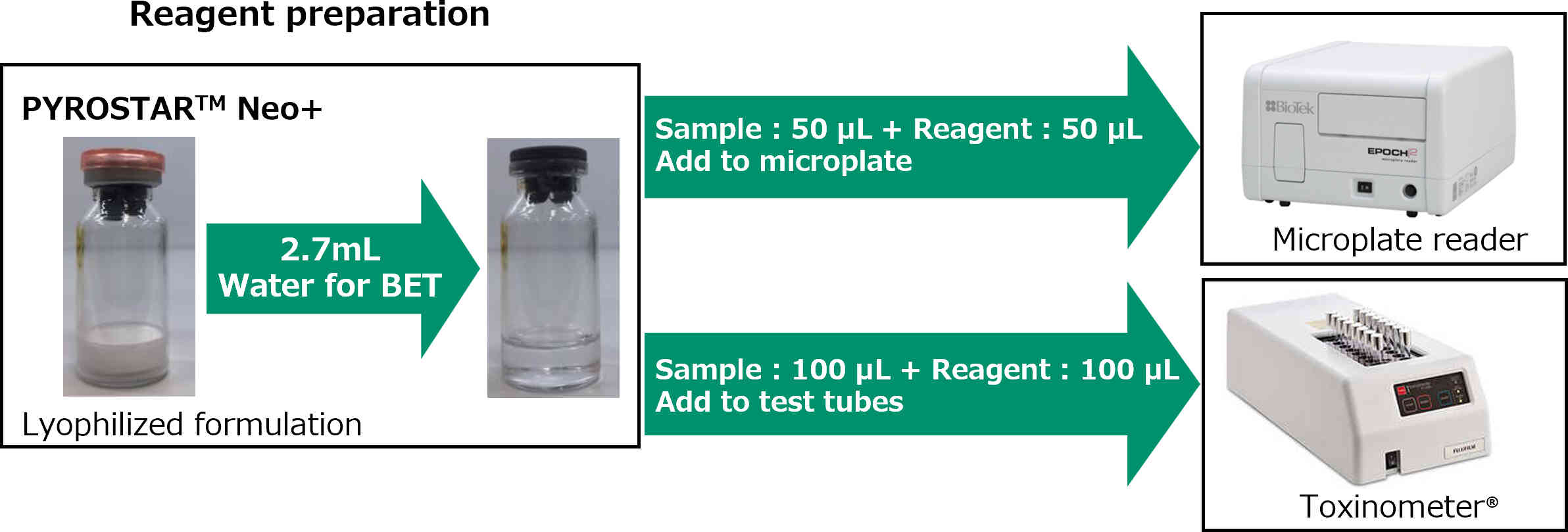
Standard Curve
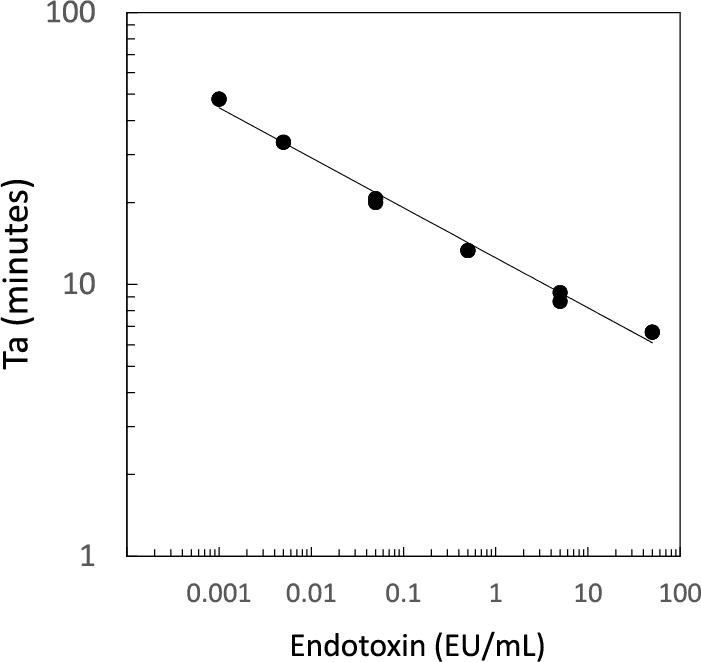
Create standard curve using the activation times obtained from the standard endotoxin. Calculate the endotoxin concentration of the sample from the activation times of the sample and the standard curve. X-axis is the endotoxin concentration on logarithm and Y-axis is the activation time on logarithm. Linear or quadratic regression can be used.High sensitivity
Quantitative range: 0.001 - 50 EU/mL
Experimental Data
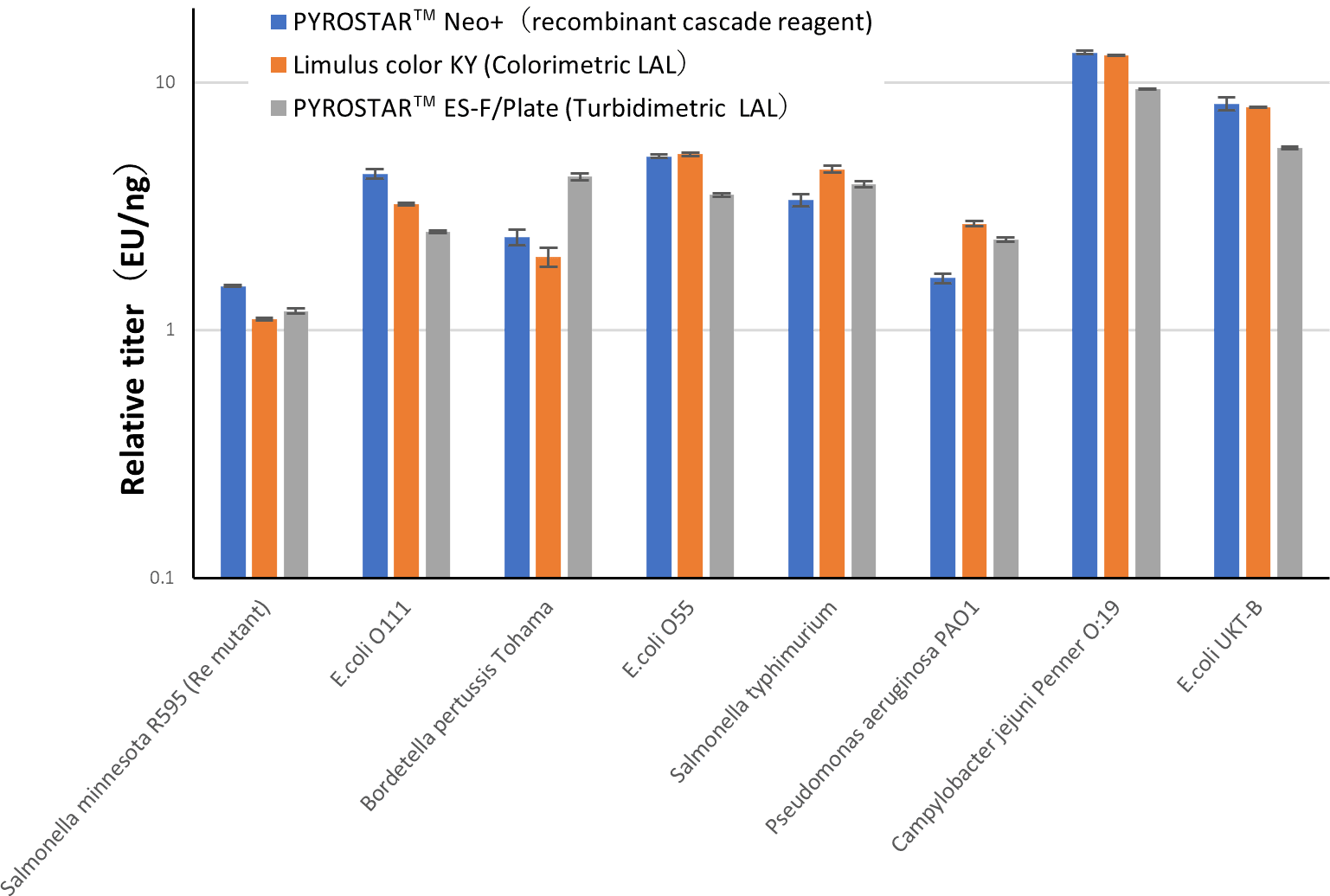
The relative titer of LPS from various bacterial species against USP-RSE measured with PYROSTAR™ Neo+ was equivalent to the titer measured with LAL reagents.
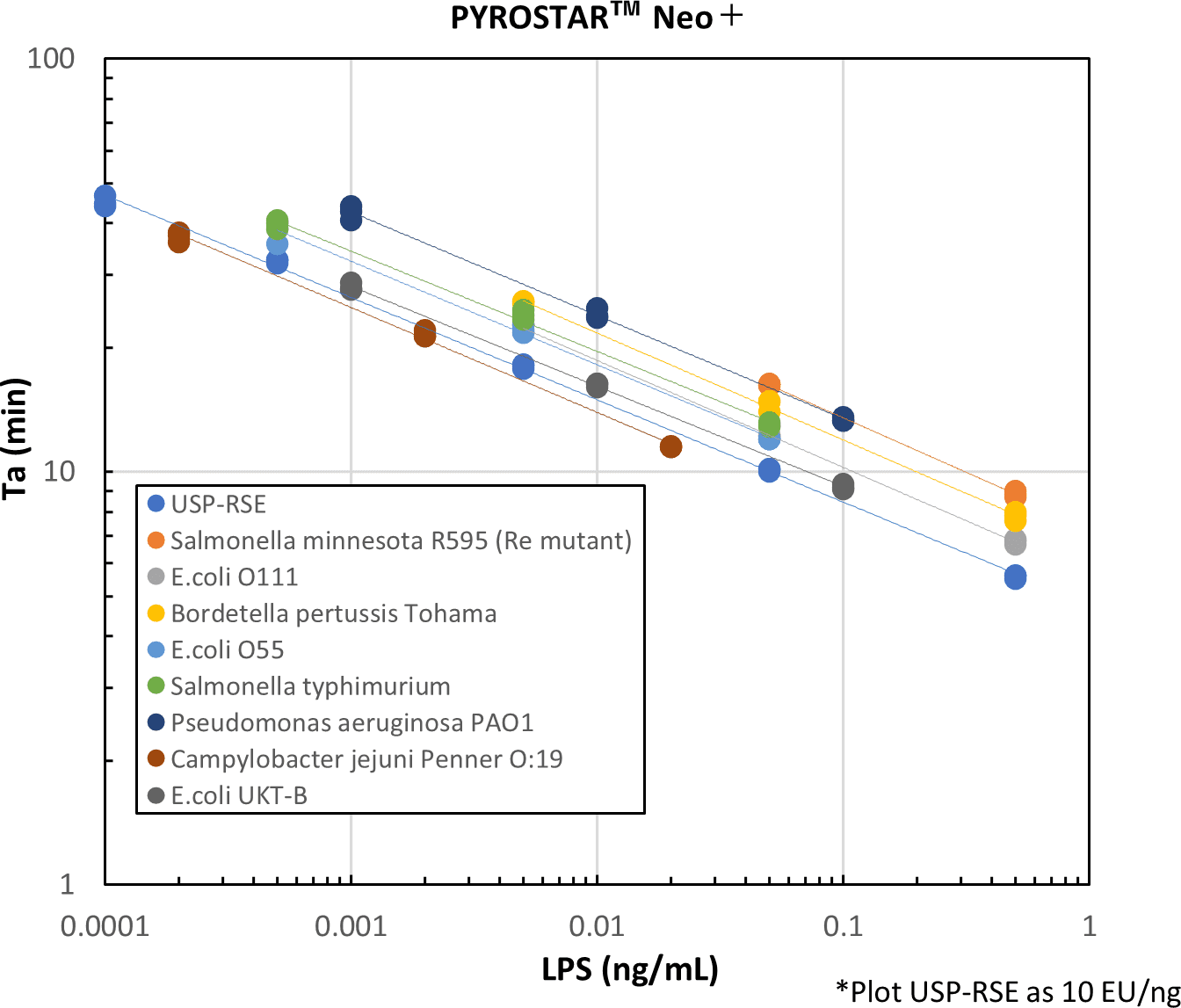
It also showed good parallelism with USP-RSE.
PYROSTAR™ Neo+ can measure not only standard endotoxin but also LPS from various bacterial species with good equivalency to LAL reagents.
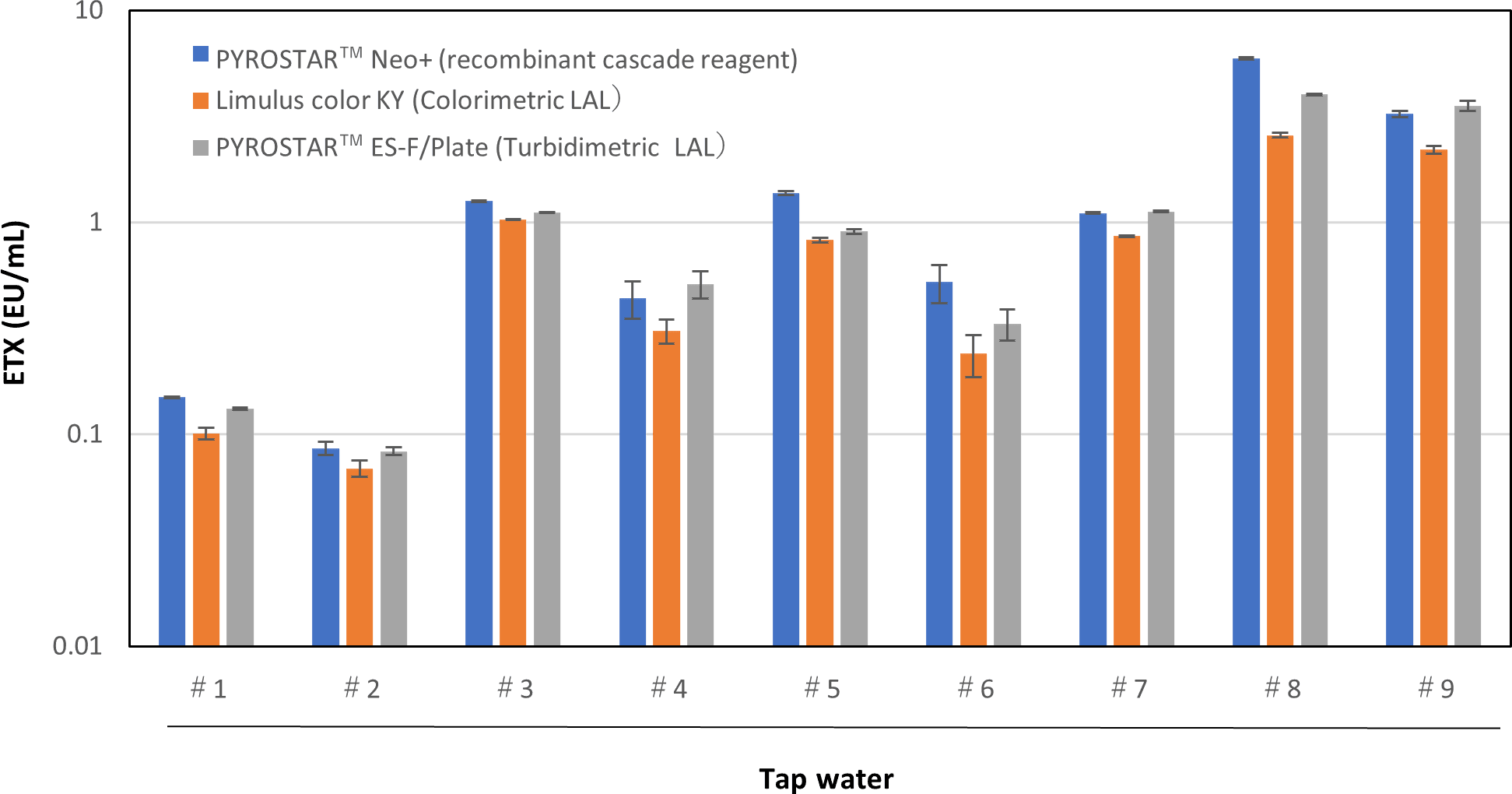
PYROSTAR™ Neo+ has equivalent reactivity against NOE compared to LAL
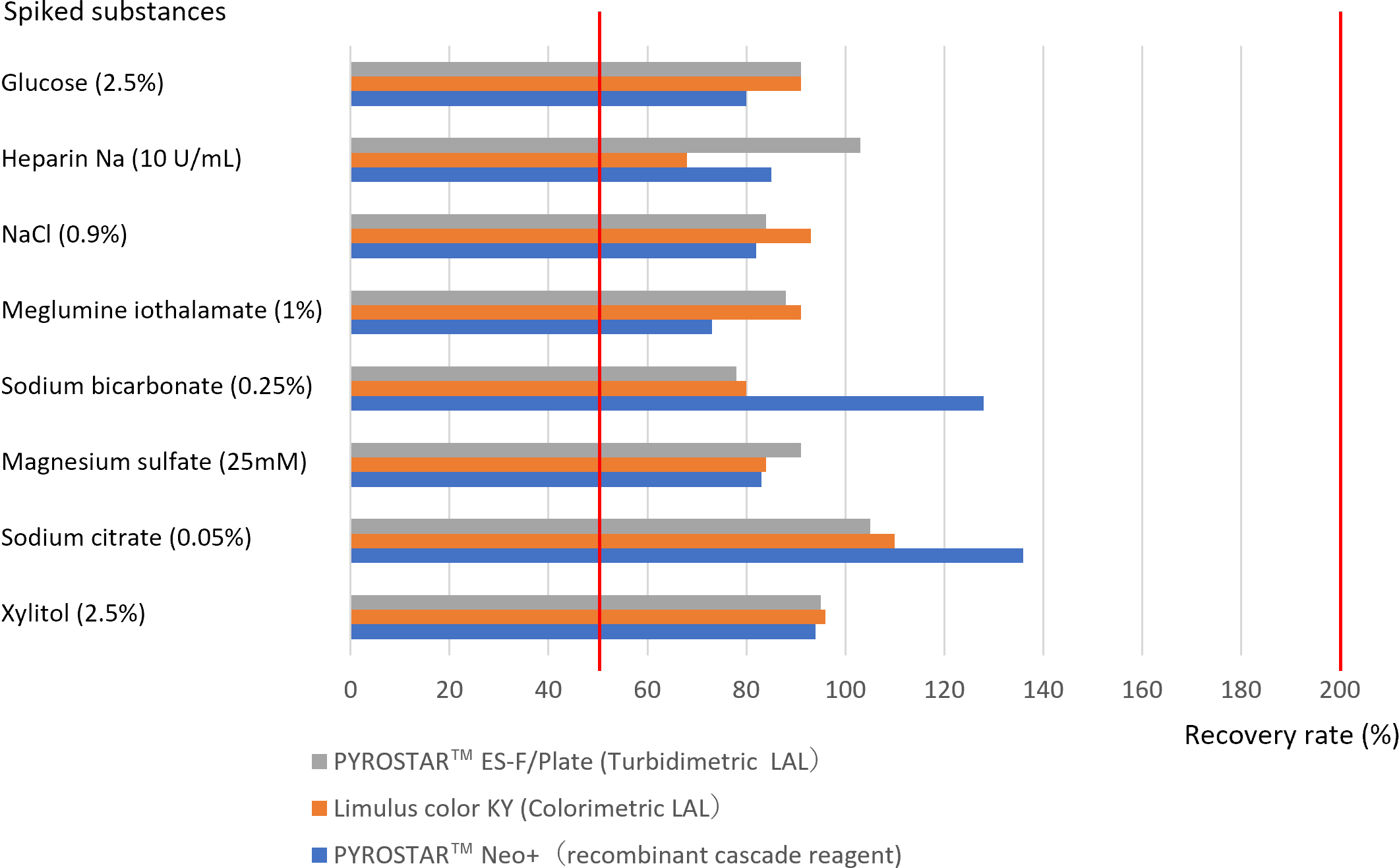
PYROSTAR™ Neo+ showed good recovery rate as well as LAL.
Product Information
| Product code | Product name | Size |
|---|---|---|
| 293-36941 | PYROSTAR™ Neo+ | 50 Tests* (for 2.7 mL) |
*For microplate reader : 50 tests
For Toxinometer™ : 25 tests