5th review: Reverse translational research in psychiatry using human blood to predict brain microglial activity
This article was written by Takahiro A. Kato, MD, PhD, Department of Neuropsychiatry, Graduate School of Medical Sciences, Kyushu University.
Introduction
While the involvement of microglia, immune cells that play an important role in brain inflammation, has recently been suggested not only in neurodegenerative diseases but also in various other neuropsychiatric disorders, relevant findings in humans are limited. Traditional clinical research focused on microglia has been mainly conducted in postmortem brain studies and positron emission tomography (PET) studies. These studies have reported microglial overactivation in the brains of patients with schizophrenia, depression, and autism spectrum disorder as well as suicide victims. Microglial activation in the brains of living patients can be measured using PET technology, but only partially, including the measurement of translocator protein (TSPO), at present, in contrast to the multifaceted nature of microglial activation. In addition, the resolution is another problem, precluding the use of PET alone to evaluate dynamic and multifaceted microglial activation, especially at the molecular level. This limitation is unignorable especially in drug discovery, in which molecular elucidation is essential.
It is ideal, but ethically and technically impractical to collect and analyze fresh microglial cells from the brains of patients who live. To elucidate the microglial pathology, therefore, it is essential to analyze fresh microglial cells from the brains of model animals such as rodents, and model mouse experiments focused on microglia have been promoted in our laboratory.1) However, it is impossible to create an animal model that can represent all phenotypes of each psychiatric disorder. To overcome these limitations, we have promoted reverse translational research on psychiatric disorders in humans using blood, which is highly accessible, as described below.
Development and application of human blood-derived iMG cells
The development of human induced pluripotent stem (iPS) cell-derived microglia-like cells has been sequentially reported since 2016, raising hopes that microglial pathology in various brain diseases can be understood.2,3) Analysis of iPS cell-derived microglia-like cells is particularly useful in genetically influenced diseases, whereas analysis of disease model cells reflecting the disease state is important in psychiatric disorders, in which environmental factors are generally more influential than genetic factors. Therefore, our laboratory has developed technology for inducing microglia-like cells directly from somatic cells rather than from iPS cells, in which disease state information has been reset. In 2014, we successfully generated directly induced microglia-like (iMG) cells in only 2 weeks by administering two cytokines, granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-34, to human peripheral blood monocytes (Fig. 1).4) Unlike iPS cells, iMG cells can be generated by chemical induction in a very short time without gene recombination, allowing relatively inexpensive production of many samples. In addition, iMG cells, which can be measured for dynamic functions such as phagocytosis and cytokine production and analyzed at various molecular levels in living cells, is expected to compensate for the disadvantages of postmortem brain and PET studies.4,5) At the time of development in 2014, iMG cells were demonstrated to share many properties with human brain microglial cells and have a different phenotype from those of monocytes and macrophages.4) A research group at Massachusetts General Hospital (MGH) in the US found by microarray analysis that iMG cells prepared based on our advice were most similar to human brain microglia.6) In cooperation with Department of Neurosurgery, Kyushu University Hospital, we also found by global gene expression analysis using RNAseq that peripheral blood-derived iMG cells were similar to concurrent brain microglia in the same patients (Tanaka S. et al.: Front. Immunol., 29 June 2021).
Human iMG cells have already been applied to understand the pathology of neuropsychiatric disorders and pain-related diseases, and to research and develop biomarkers. Our initial focus was placed on Nasu-Hakola disease, which is known as a representative primary microglial disease. In iMG cells prepared from a female patient who developed psychotic symptoms in her 30s and became bedridden with progressive dementia symptoms in her 40s, phagocytosis-stimulated production of inflammatory cytokines such as tumor necrosis factor (TNF)-α and IL-6 tended to be delayed, and the production of protective cytokines such as IL-10 tended to be suppressed from an early stage onwards.4) These reactions of patient-derived iMG cells suggest the involvement of microglia-induced chronic brain inflammation in the development of various psychiatric symptoms and premature dementia in Nasu-Hakola disease.4) Subsequently, iMG cells from patients with type I or rapid cycling bipolar disorder whom I clinically took care of were analyzed. One male patient showed a M1-dominant mRNA profiling during mania, and an analysis of 3 patients performed with cooperation from Department of Psychiatry, Saga University Hospital revealed increased mRNA expression of the mannose receptor CD206, a representative marker of M2-dominant polarization, during depression phase in all these patients.7) These results suggest that changes in microglial immune responses may play an important role in a shift from mania to depression or vice versa in bipolar disorder.7) In addition to psychiatric disorders, we have been engaged in reverse translational research with the use of iMG cells from patients with physical diseases5) in cooperation with Department of Psychosomatic Medicine, Kyushu University Hospital, and found high expression of TNF-α mRNA in iMG cells of female patients with fibromyalgia, proposing that this may be an objective biomarker of pain-related disorders.8) Our collaboration with Department of Neurosurgery, Kyushu University Hospital has also led to an emerging finding that the expression pattern of CD206 on iMG cells may be a predictive marker for progression of brain tumor (glioma) (Tanaka S. et al.: Front. Immunol., 29 June 2021). iMG cells have already been used in research institutions outside Japan as well. In co-culture experiments of blood-derived iMG cells from patients with schizophrenia and iPS cell-derived neurons from the same patients, the aforementioned MGH group reported that microglial activation was important for complement-mediated neuronal synaptic damage in patients, and that neuronal synaptic damage was prevented by minocycline, an antibacterial that inhibits microglial activation.9) A joint study team of researchers in the world, including the US and the Netherlands, has also started research using schizophrenia patient-derived iMG cells, and reported that patient-derived iMG cells were more responsive to lipopolysaccharide (LPS) and produced more TNF-α.10)
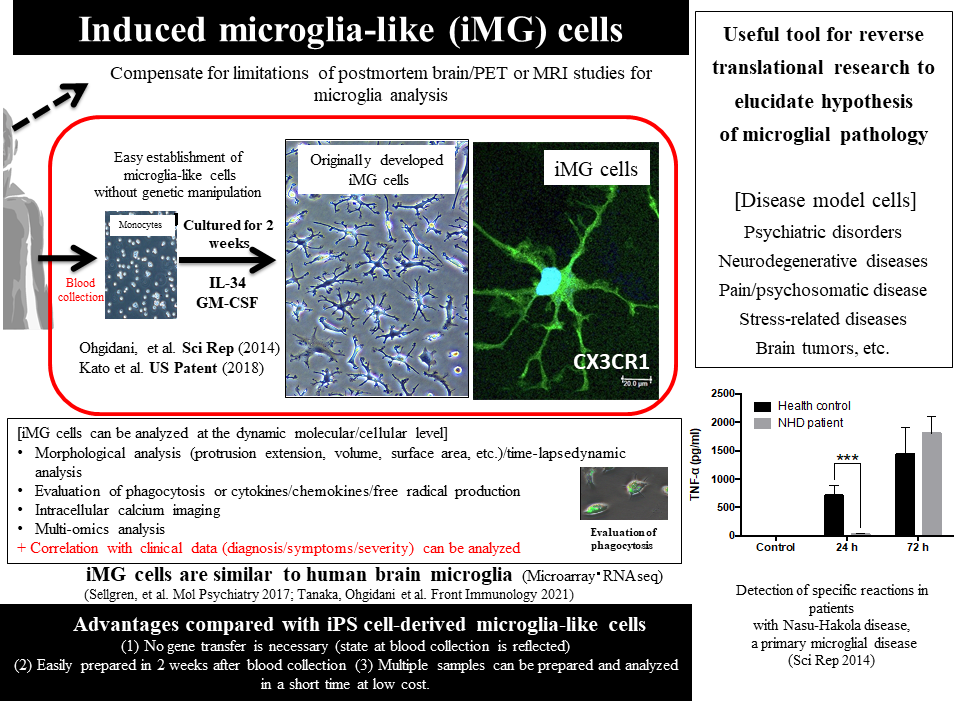
Figure 1: Features of human blood-derived directly induced microglia-like (iMG) cells (Revised from References 4 and 5)
Human blood metabolome analysis
In my laboratory, not only blood cell components, but also plasma and serum are used to find a clue to understanding microglial dynamics in the brain. For instance, we have used plasma for alternative analysis of neuron-derived exosomes and found that IL-34, a cytokine essential for microglial activity, may be involved in the pathology of depression in acute phase.11) In collaboration with the Department of Clinical Chemistry and Laboratory Medicine, Kyushu University Hospital, on the other hand, peripheral blood was collected from patients with depression for metabolome analysis, leading to the identification of several blood metabolites associated with the severity of depression and the finding that the intensity of suicidal ideation correlated with multiple metabolites in the tryptophan-kynurenine metabolic pathway, which is closely associated with microglial activation.12,13) These results support microglial activation in the brains of suicide victims in postmortem brain studies. Based on the belief that the control and understanding of microglial activation are also useful in preventing suicide, which is an urgent social problem, therefore, we are currently engaged in interactive research by combining various methods14) (Fig. 2).
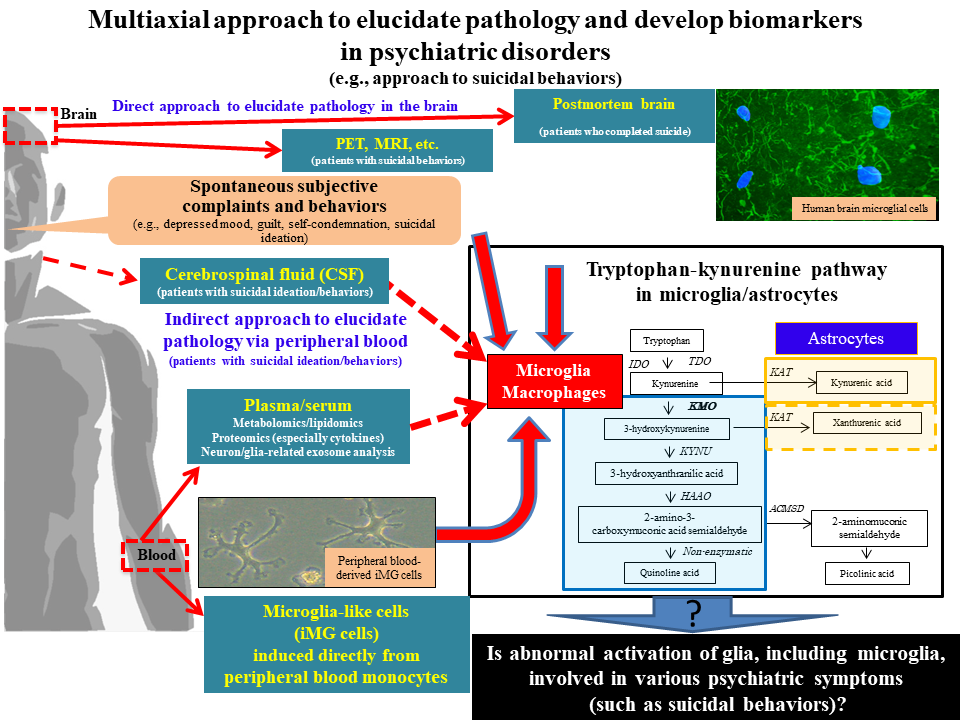
Figure 2: Multiaxial approach to elucidate pathology and develop biomarkers in psychiatric disorders (e.g., approach to suicidal behaviors), cited/revised from References 14
Conclusion
Excessive or uncontrollable microglial activation may be involved in various psychiatric symptoms, including depression, anxiety, delusion, and suicidal ideation. In the future, reverse translational research combining clinical data collection, human blood analysis (analysis of iMG cells and metabolome analysis), and human brain analysis in various psychiatric disorders is expected to evolve to elucidate the microglia-mediated mechanism of psychiatric pathology at the molecular/cellular level, leading to the development of new microglia-targeting drugs. Both analysis of iMG cells, which can be prepared from collected blood, and metabolome analysis are expected to be used as objective biomarkers reflecting microglial dynamics in the brain not only in psychiatric disorders, but also in various other physical diseases.
References
- Ohgidani, M. et al. : Brain Behav. Immun., 55, 17-24 (2016).
- Muffat, J. et al. : Nat. Med., 22, 1358-1367 (2016).
- Pocock, J. M. and Piers, T. M. : Nat. Rev. Neurosci., 19, 445-452 (2018).
- Ohgidani, M. et al. : Sci. Rep., 4, 4957 (2014).
- Ohgidani, M. et al. : Front. Cell. Neurosci., 9, 184 (2015).
- Sellgren, C. M. et al. : Mol. Psychiatry., 22, 170-177 (2017).
- Ohgidani, M. et al. : Front. Immunol., 7, 676(2017).
- Ohgidani, M. et al. ; Sci. Rep., 7, 11882 (2017).
- Sellgren, C. M. et al. : Nat. Neurosci., 22, 374-385 (2019).
- Ormel, P. R. et al. : Brain Behav. Immun., 90, 196-207 (2020).
- Kuwano, N. et al. : J. Affect. Disord., 240, 88-98 (2018).
- Kuwano, N. et al. : J. Affect. Disord., 231, 74-82 (2018).
- Setoyama, D. et al. : PLoS One, 11, e0165267 (2016).
- Suzuki, H. et al. : Front. Cell. Neurosci., 13, 31 (2019).




