Introduction of RAFT agents that can be mass-produced
Living radical polymerization
Radical polymerization is a type of polymerization in which neutral and highly active carbon radical species are utilized. Alkenes (olefins and vinyl compounds) are most often used as the monomer, and the compounds that form radicals in thermal, optical, or catalytic processes act as initiators. In addition to its wide applicability making it possible to polymerize most vinyl compounds, radical polymerization has the ability to deliver polymers with high molecular weights relatively more easily under milder reaction conditions than ion polymerization or sequential growth polymerization (polycondensation, polyaddition), or even in water in some cases.
On the other hand, it is difficult to control the reaction, due to the occurrence of stops caused by "rebinding", in which the propagating radicals bind with one another, and side reactions such as "chain transfer" occur, in which the propagating radicals deactivate as they extract the hydrogen from other molecules in the reaction system and generate new propagating radicals. As the growth reactions continue, it is difficult to control the molecular weight precisely once an activated radical species has been formed unless a stop reaction or chain transfer reaction occurs.
Yet, it is possible to temporarily convert the propagating radical species into inactive dormant species and suspend the growth of the polymer chain in a method that forms the radical species in a reversible manner. When the conversion reaction between the dormant species and the radical species occurs rapidly, growth reaction occurs in all the polymer chains in the reaction system at the same rate, resulting in formation of polymers of uniform molecular weight. Furthermore, side reactions can be suppressed by shifting the equilibrium to the dormant side and reducing the concentration of the radical; this specifies when the dormant and radical species are in equilibrium.
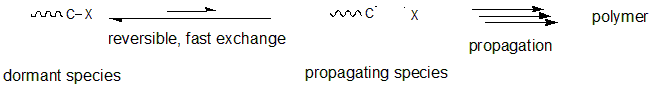
Fig.1 Reaction mechanism of living radical polymerization
Various types of living radical polymerization
Various types of living radical polymerization have been reported in the 1990s and later. The polymerization methods that are widely used at present include the method in which the C-ON bond is reversibly activated by heat to provide nitroxide, a stable radical. The method in which the carbon-halogen bond is activated by single-electron oxidation-reduction reaction using a transition metal catalyst. And the method in which the thioester bond is activated by a carbon radical species to promote polymerization in a reversible chain transfer.
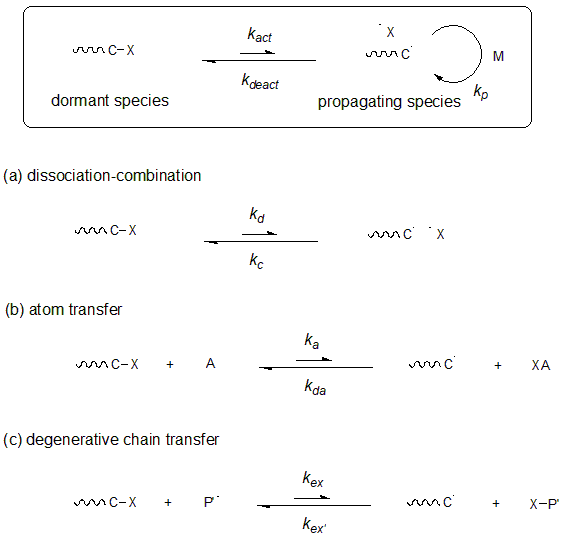
Fig.2 Classification by the mechanism of living radicals
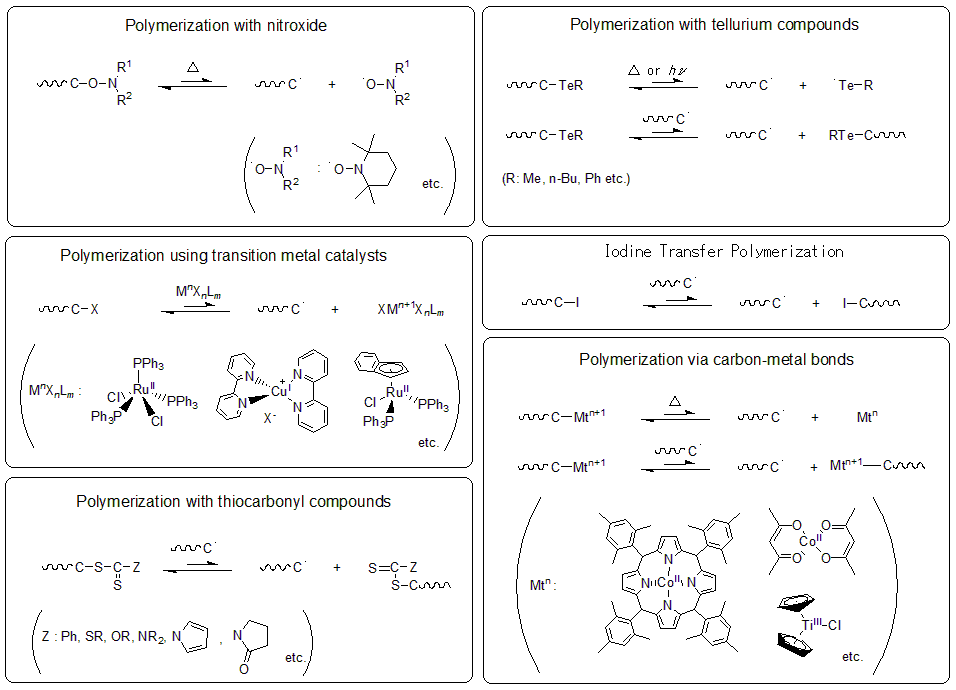
Fig.3 Some radical polymerization using various chemical species
Living radical polymerization using nitroxide (NMP)
Living radical polymerization using a nitroxide radical (while its official name is nitroxyl radical, we will use the term nitroxide radical in this article, as this name has been conventionally used in this field) was first reported as the radical polymerization of styrene using 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), which is a typical stable nitroxide radical. In this report, it was shown that polymers with a relatively narrow range of molecular weight distribution, around several tens of thousands (Mw/Mn<1.3), could be produced in polymerization under conditions where TEMPO was added to the radical polymerization system for styrene using benzoyl peroxide as the initiator. Thanks to the molecular design of nitroxide, it has become possible today to apply this to a wide range of monomers, primarily acrylic esters.
Since alkoxyamine compounds, which are dormant specifies, are stable and can be isolated, they have been widely used in research on precision polymerization synthesis as a "single molecule initiator" for living radical polymerization.
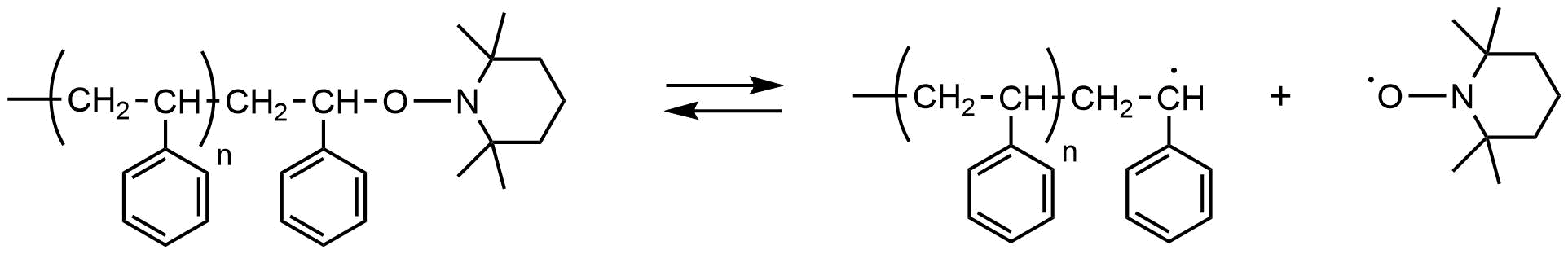
Fig.4 Living radical polymerization of styrene using TEMPO (NMP)
Living radical polymerization using metal catalysts
Living radical polymerization methods that use transition metals that undergo single-electron oxidation-reduction in radical polymerization can be roughly classified into two groups. One involves a mechanism in which the growth terminal is capped with a dissociable group such as a halogen. This dissociable group moves between the growth terminal radical and the metal while going through single-electron oxidation-reduction of the metal complex (Mn < - > Mn+1). This is called atom transfer radical polymerization. The second group involves the metal being directly attached to the growth terminal as a dissociable group, where the single-electron oxidation-reduction of the metal causes the formation of propagating radical species. This is called organometallic-mediated radical polymerization.
Although the presence of metal residues in the produced polymer may be problematic depending on the application, a system that can control polymerization with a very small quantity of metal catalyst has recently been developed8. Metal-free polymer synthesis is preferred for applications such as electronic materials and biomedical applications. In recent years, metal-free organocatalyzed atom transfer radical polymerization (O-ATRP), which uses organic photocatalysts, has also been reported9.
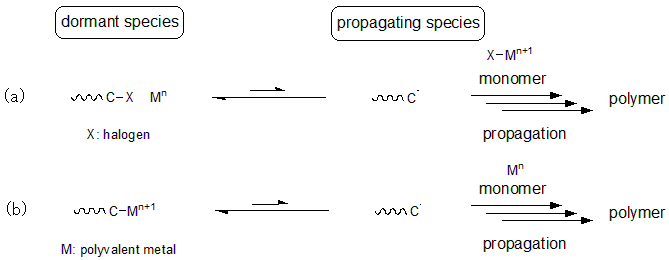
Fig.5 (a)Atom Transfer Radical Polymerization (ATRP) and (b)Organometallic Radical Polymerization (OMRP)
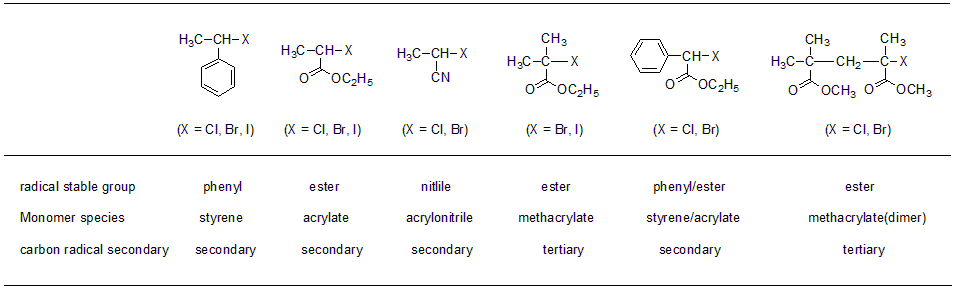
Fig.6 Example of ATRP initiator
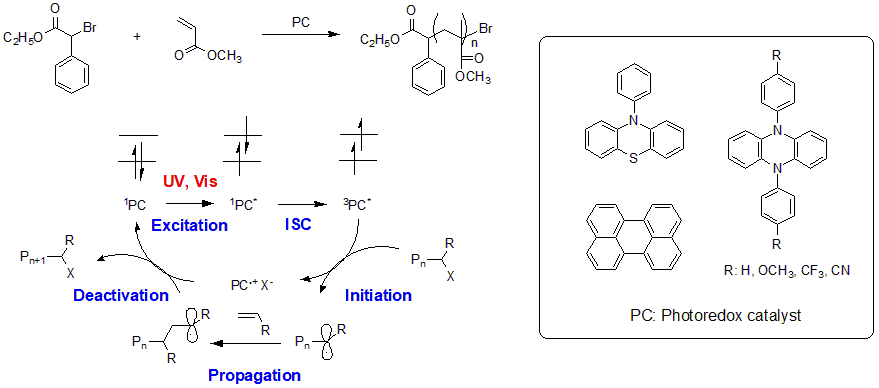
Fig.7 Example of ATRP method (O-ATRP method) using organic photocatalyst
Living radical polymerization that progresses by reversible chain transfer
In this reaction system, a polymerization terminal radical is added to the carbon-sulfur double bond in the thioester. The polymerization continues when a new activated radical and a dormant species are formed by way of an intermediate radical. This mechanism is called reversible addition-fragmentation chain transfer polymerization.
In order to obtain polymers with a narrow molecular weight distribution, it is necessary that the inactivation reaction of polymerization terminal radical is sufficiently faster than the polymerization reaction. On the other hand, sufficient activation reaction is required in order for the reaction to proceed quickly. Although these two factors are in a trade-off relationship in a reversible stop mechanism, the key feature of the reversible chain transfer is that both can be facilitated at the same time. Studies in various fields, as well as industrial applications, are being actively examined for RAFT polymerization owing to its characteristics, including simplicity in enabling polymerization by only adding a chain transfer agent (RAFT agent) to the existing polymerization reaction system without any change in facilities, and the fact that it does not use metals or halogens.
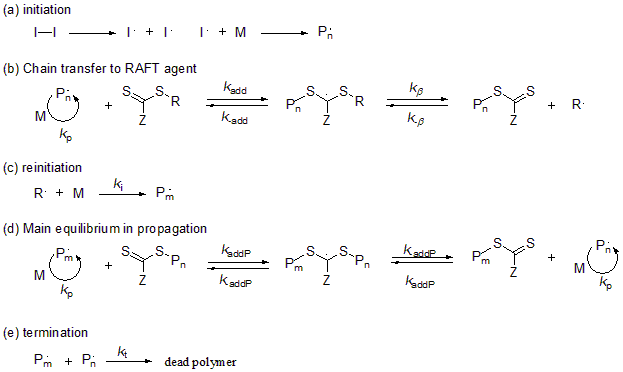
Fig.8 Reaction mechanism of reversible addition-cleavage chain transfer (RAFT) polymerization




