Novel Tool Box for EVs Therapy in Regenerative Medicine
MSC-derived extracellular vesicles isolated by PS affinity method have high anti-inflammatory and anti-fibrotic effects.
Extracellular vesicles (EVs) are small and lipid bilayer particles released by cells. EVs have now been recognized as crucial messengers in intercellular communication via the transfer of bioactive lipids, proteins, and nucleic acids. EVs derived from mesenchymal stem cells (MSC) are emerging as a promising therapeutic strategy in regenerative medicine. Here we introduce our EVs isolation technology - PS affinity method, and the usefulness of it in the regenerative medicine, which we have discovered recently.
Extracellular vesicles (EVs)
EVs, including exosomes and microvesicles, are lipid bilayer particles smaller than 1,000 nm and released by cells and they have CD9, CD63, and CD81 as marker protein1). EVs contain cellular components such as protein, DNA, RNA, and lipid. They are considered to be related to intercellular communication via the transfer of these components2).
MSC-derived EVs
MSC is a mesenchymal-derived stem cell and it can differentiate into adipose, bone, and cartilage. As MSC can be established from bone marrow, adipose tissue, or umbilical cord, it is a promising source of regenerative medicine. MSC is known to have some paracrine effects including immune suppression, anti-inflammation, and anti-fibrosis. Recently it has been reported that these effects are caused by EVs released from MSCs3). That is why MSC-derived EVs therapy has been getting attention.
Novel EVs isolation technology -PS affinity method-
To isolate EVs, there are some methods including ultracentrifugation which has been used for a long time, density gradient centrifugation, and antibody affinity method against the surface antigen4). However, those methods have some problems such as low recovery efficiency, low purity, inability to collect intact vesicles, and poor reproducibility. We have developed the new PS affinity method which captures phosphatidylserine (PS) expressing on the surface of EVs in cooperation with Prof. Hanayama from Kanazawa University5). PS affinity method utilizes Tim4 protein which binds to PS on calcium dependency. Although Tim4 protein binds to PS strongly, the binding is released by Ca2+ chelate agents such as EDTA. Utilizing these properties, we could develop the EVs isolation method which has better recovery efficiency, purity, collection of intact vesicles, and reproducibility than the conventional methods (Fig.1).
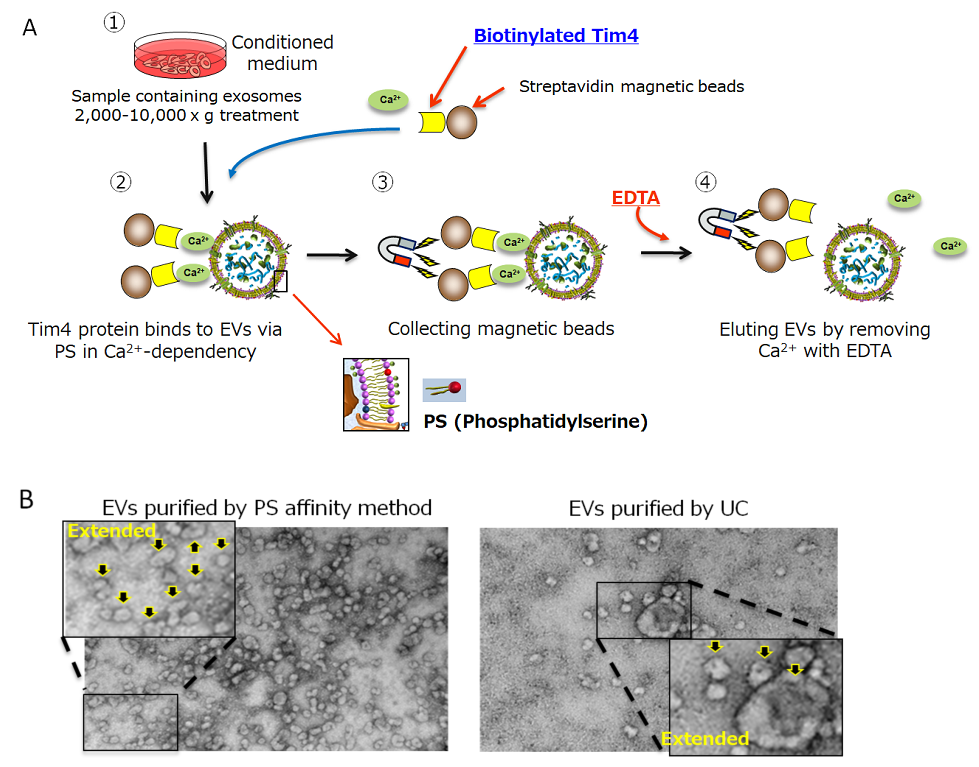
Figure 1.
A) EVs isolation procedure by PS affinity method
B) Transmission electron microscope figures of EVs isolated by PS affinity method or UC. Arrow indicates EVs.
PS affinity method enables isolation of MSC-derived EVs with high activity
We introduce the values of the PS affinity method on the isolation of MSC-derived EVs, which we have discovered recently. Firstly, we isolated EVs from the conditioned medium of bone marrow derived-MSC by PS affinity method or ultracentrifugation method (UC). Then we compared the recovery efficiency of each isolation method by PS ELISA which is the EVs quantitative method using PS affinity technology (Figure 2A). We analyzed the quantitation of EVs by detecting CD9, CD63, or CD81. As a result, UC showed 40% recovery rate, while the PS affinity method showed more than 80% recovery rate (Figure 2B).
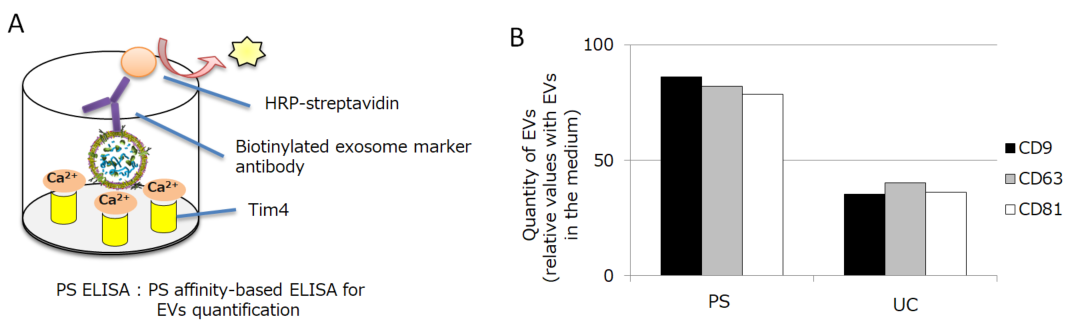
Figure 2.
A) Principle of PS ELISA
This is our original technology to quantify EVs. It captures EVs by immobilized Tim4 protein and detects the surface antigen of EVs by the detection antibody.
B) Quantification of MSC-derived EVs isolated by PS affinity method or UC method
The detection antibody was either anti-CD9 antibody, anti-CD63 antibody, or anti-CD81 antibody. The graph shows the relative values with the amount of EVs in the medium being 100%.
PS ELISA method: PS Capture™ Exosome ELISA Kit (Streptavidin HRP) (298-80601; Anti CD63-antibody is included), Anti-CD9 antibody (019-27953), Anti-CD81 antibody (011-28111)
Besides, we compared the activities of MSC-derived EVs isolated by each method. We conducted the experiments to review the anti-inflammatory effect with peripheral blood mononuclear cells (PBMC) and anti-fibrotic effect with normal human fetal lung diploid fibroblast cells (TIG3 cells). The results showed that the MSC-derived EVs isolated by PS affinity method have much higher activities than the EVs isolated by UC (Figure 3, 4).
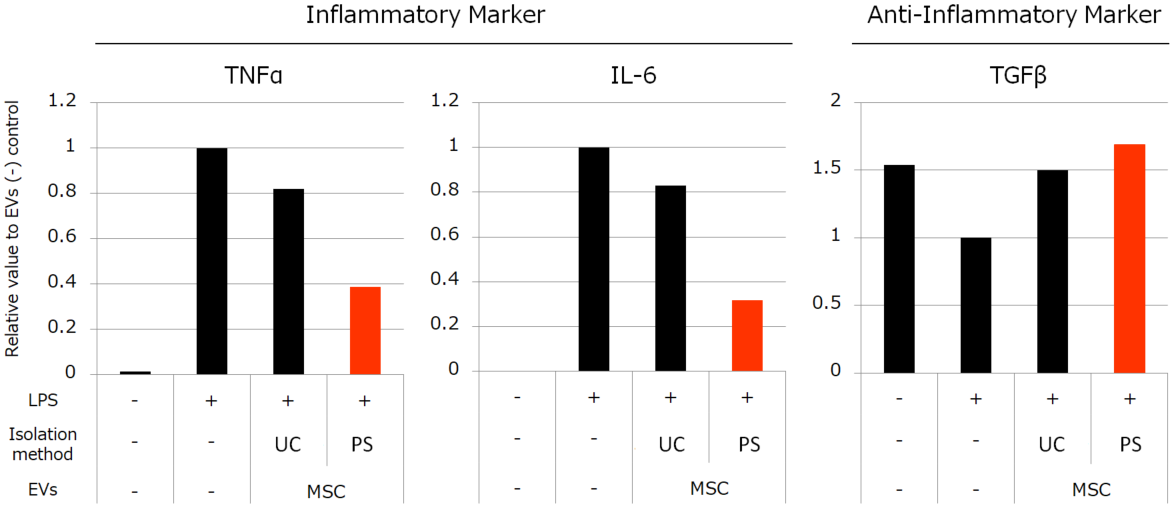
Figure 3. Effects of MSC-derived EVs to inflammation induced by LPS
We stimulated monocytes isolated from PBMC with LPS to induce inflammation. Then we added 4.5×108 particles/mL EVs isolated by each method. We found that MSC-derived EVs suppressed the elevation of TNFα and IL-6 gene expression and the reduction of TGFβ. The suppressive effects were induced more strongly by EVs isolated by PS than EVs isolated by UC.
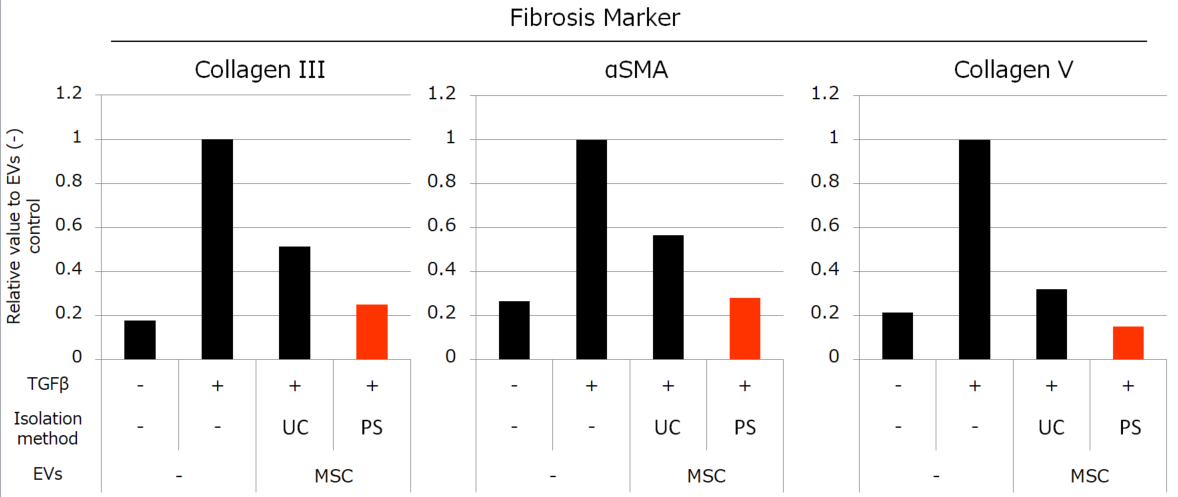
Figure 4. Effects of MSC-derived EVs to fibrosis
We stimulated TIG3 cells with TGFβ and induced the increased expression of fibrosis-related genes, then added 1×109 particles/mL of EVs isolated by each method. MSC-derived EVs suppressed the elevation of Collagen III, αSMA, and Collagen V genes. The suppressive effects were induced more strongly by EVs isolated by PS than EVs isolated by UC.
These results show that the PS affinity method can isolate MSC-derived EVs with high recovery efficiency while maintaining the high activity of them. In other words, it revealed that the conventional method harmed the functions of EVs by physical damage of ultracentrifugation in addition to the low recovery efficiency. Our PS affinity method has the potential to be an innovative technology for future EVs therapy.
Conclusion
As we said earlier, while EVs-based therapy in regenerative medicine has been getting attention, the classical method such as ultracentrifugation has still been used widely to isolate EVs6). Our PS affinity method is an ideal method which solves the conventional methods' problems such as low recovery efficiency and reducing activity of EVs. Our kit has superior recovery efficiency, purity, ability to collect intact EVs, and reproducibility. It is supposed that the optimized EVs isolation method will be required for regenerative medicine, and we hope our PS affinity method would be a powerful tool for that.
Products used in the article
| Product No. | Product Name | Package Size |
|---|---|---|
| 299-77603 | MagCapture™ Exosome Isolation Kit PS | 2 Tests |
| 293-77601 | 10 Tests | |
| 297-79201 | PS Capture™ Exosome ELISA Kit (Anti Mouse IgG POD) | 96 Tests |
| 298-80601 | PS Capture™ Exosome ELISA Kit (Streptavidin HRP) | 96 Tests |
Video
- Seminar at ISEV 2020
[Characteristics on PS Affinity for Isolation and Detection of Mesenchymal Stem Cell-derived EVs]
How to use MagCapture™ Exosome Isolation Kit PS
Product Literature
References
- Colombo et al., Biogenesis, secretion, and intercellular interactions of Extracellular vesicles and other extracellular vesicles. Annu Rev Cell Dev Biol, 30 : 255-289 (2014).
- Mathieu et al., Specificities of secretion and uptake of Extracellular vesicles and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol, 21(1) : 9-17 (2019)
- Phinney and Pittenger, Concise Review : MSC-Derived Extracellular vesicles for Cell-Free Therapy. Stem Cells, 35(4) : 851-858 (2017)
- Thery et al., Isolation and characterization of Extracellular vesicles from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chapter 3, Unit 3 22 (2006)
- Nakai et al., A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci Rep 6 : 33935 (2016)
- Fujita et al., Clinical Application of Mesenchymal Stem Cell-Derived Extracellular Vesicle-Based Therapeutics for Inflammatory Lung Diseases. J Clin Med 7(10) : 355 (2018)





