The Story of the Development of CTX-ELISA 1B
The original article was written by Masahiro Hirama, Professor Emeritus of Tohoku University, and Acroscale Ltd. (Glytech, Inc.) for Vol. 07 (December 2018) of ChemGrowing.
This article has been translated from the above Japanese version by Fujifilm Wako.
Background
Scientific research, chemistry in particular, originates from "curiosity and ambition" to "create or discover" "something interesting," and makes great progress when "time, advantage of position, and human relationship" work well together. It was almost 30 years ago when we launched an endeavor to develop a kit to detect ciguatoxins by producing anti-ciguatoxin antibodies. Just at the time I started a new research laboratory and intended to do new total synthesis research that would lead to benefit humankind. Thanking to many collaborators, I herein look back at how our endeavor came to fruition as CTX-ELISA 1B.
Overview
The toxicity of ciguatoxin (Figure 1), a causative neurotoxin of ciguatera marine fish poisoning (CFP) that occurs in subtropical and tropical regions is nearly one hundred times more toxic than the pufferfish tetrodotoxin. More than 50,000 people worldwide suffer annually from CFP.1-3) CFP occurs in Japan mainly in Okinawa, but recently sometimes near the Kanto region.
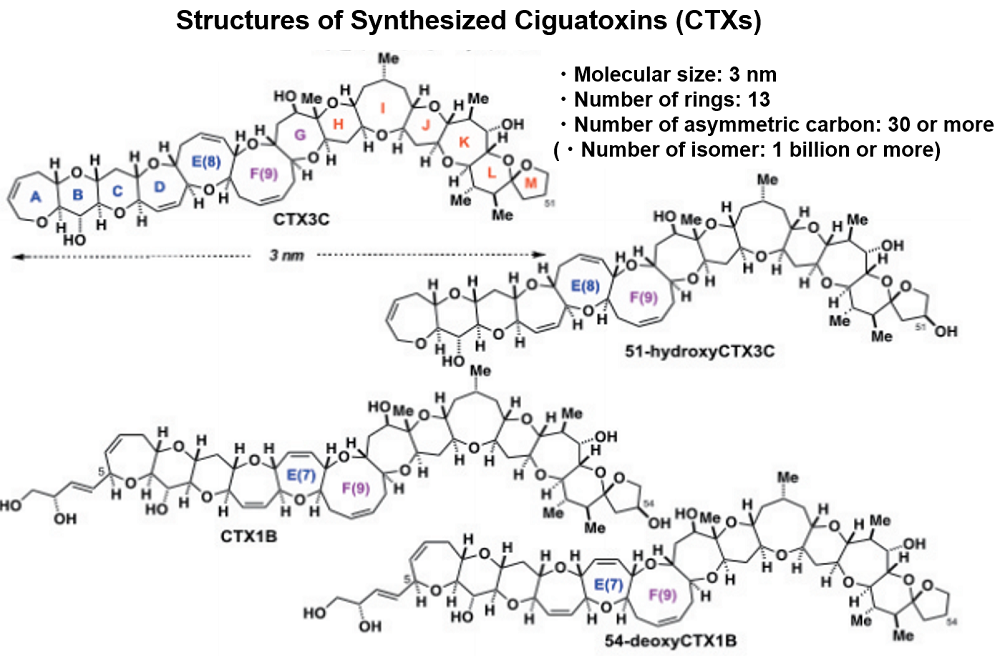 Figure 1: Structures of Synthesized Ciguatoxins (CTXs)
Figure 1: Structures of Synthesized Ciguatoxins (CTXs)
 Figure 2: Fish often cause ciguatera poisoning (CFP)
Figure 2: Fish often cause ciguatera poisoning (CFP)
In 1989, Prof. Takeshi Yasumoto, Professor of the Faculty of Agriculture, Tohoku University, in collaboration with researchers in Tahiti, isolated 0.35 mg of ciguatoxin CTX1B from 4 tons of moray eel and determined its structure using state-of-the-art NMR.4) They also showed that ciguatoxins are produced not by fish but by plankton dinoflagellates, hence ciguatoxins get accumulated in carnivorous fish in the food chain (Figure 2).1) CFP is caused by the ingestion of a variety of reef fish that have accumulated trace amount of ciguatoxins. Ciguatoxins are tasteless, odorless, and could not be broken down even when boiled or baked. The total amount of ciguatoxins in one fish is very small, so that it is not lethal fortunately, but patients often fall in deathly ill. Symptoms of CFP begin with diarrhea and vomiting, followed by reduced heart rate and blood pressure, and then by neurological disorders such as dry ice sensation, that may last for several months to more than a year. Thus, it is very serious for the patients. However, there were no effective simple detection or treatment method for ciguatoxins.2-5)
Progress of Total Synthesis of Ciguatoxins and Attempts to Produce Antibodies
In early 1989, we received an offer from Asst. Prof. Dr. Michio Murata (now Professor at Osaka University) in Yasumoto laboratory, who was conducting structural research using the state-of-the-art NMR. He asked me to synthesize a putative substructure of ciguatoxin to confirm the partial structure. It was the beginning of our synthetic endeavor on ciguatoxins. However, we were not able to contribute to the structural study because our synthesis ability at that time was very limited. Within six months, Prof. Yasumoto and Dr. Murata determined the structure of CTX 1B (Figure 1).4) I was really fascinated to see the structure: a giant molecule of more than 3 nm long with 13 rings in a ladder arrangement. At the time, no one had synthesized such a large and complex molecule. We were not confident that we can accomplish the total synthesis, but we just intended to challenge it.
Dr. Hokama group of the University of Hawaii, on the other hand, had worked on developing antibodies using natural ciguatoxin 1B itself before its structure was elucidated. They claimed that they had succeeded in producing a ciguatoxin antibody by immunization of mice with a mixture of ciguatoxin, a protein, and condensation reagent, and developed a ciguatoxin detection kit called Cigua-Check. However, the kit proved to show the low specificity and thus the antibody itself has been questioned.3)
It is well known that when antibody binds to antigen the antibody does not recognize the whole structure of antigen, but instead only a part of it. We therefore expected that once a structurally defined nontoxic partial structure of ciguatoxin was synthesized, it could be usable as a hapten to produce the antibody. In 1992, we synthesized a simple AB ring model of CTX1B and bound it to bovine serum albumin (BSA) and/or oval albumin (OVA). Immediately, Prof. Yasumoto arranged the collaborative antibody-production study with the researchers of the Institut Louis Malardé of Medical Research in Tahiti. More than six months after we sent the AB ring model-antigen to Tahiti, they informed us that antibodies were produced in the serum of the mice to some extent, but they were not able to obtain monoclonal antibodies. Tahiti is too far away from Sendai to communicate quickly. E-mail was not yet available at that time.
In May 1993, Dr. Takeshi Tsumuraya, who had started a study of catalytic antibody at the Kao Research Laboratory for Basic Science, asked me to give a lecture at the Laboratory. Dr. Tsumuraya is a professional in antibody production against a small molecule. After my lecture, I asked him for collaboration in the development of ciguatoxin antibodies, and the collaborative study started. Immunization using the antigen of the AB ring model-BSA conjugate did not give good results. Dr. Tsumuraya then suggested that we should use keyhole limpet hemocyanin (KLH) instead of BSA and OVA. The three-ring (ABC) model KLH conjugate was synthesized and immunized in mice but still failed. Dr. Tsumuraya then transferred to the Research Laboratory for Biomolecular Engineering, and our collaboration was suspended temporarily. Instead, at the meeting of Tohoku University Research Network System (TURNS) Prof. Nakao Ishida, President of Tohoku University, introduced me to Prof. Michinao Mizugaki, Director of the Pharmaceutical Department of Tohoku University Hospital. Prof. Mizugaki and his coworkers helped us in developing antibodies, but our efforts made no advance.6)
Completion of Total Synthesis and Antibody Production
The dramatic progress of our research came in 2000. I got a chance to meet Dr. Ikuo Fujii of Institute of Biomolecular Engineering in the corridor of the hotel at the International Chemical Congress of Pacific Basin Societies in Hawaii at the end of December. He just came out of the conference room for a smoke break. I had previously met Dr. Fujii at a bar in Sendai and had drinks together. It was a surprise and a good fortune to see him and to hear that he was the head of the research laboratory that Dr. Tsumuraya joined. I told him about the current situation of our ciguatoxin antibody project, and he immediately offered me to help the project with Dr. Tsumuraya. At that time our total synthesis of ciguatoxin had made a great progress. In the spring of 2001, we completed the total synthesis of CTX3C using the unified convergent [X+2+Y] construction strategy (Figure 3)7) The synthetic intermediates were also proven to be useful as non-toxic haptens.
 Figure 3: Convergent total synthesis of ciguatoxin CTX3C with a two-ring construction.
Figure 3: Convergent total synthesis of ciguatoxin CTX3C with a two-ring construction.
In October 2003, Drs. Fujii and Tsumuraya moved to Osaka Prefecture University (now, Osaka Metropolitan University), and they had more freedom to conduct our collaborative study. Moreover, our funding situation got much better: Research funds from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) as well as CREST of the Japan Science and Technology Agency (JST) greatly accelerated our research. We eventually found that in order to produce highly sensitive and specific antibodies which bind strongly to ciguatoxins, the synthetic polyether haptens should possess more than five ether rings and surface area larger than 400 Å2. For example, monoclonal antibody 10C9, which specifically bound to the left half of CTX3C, was obtained by immunizing mice with the synthetic intermediate ABCDE fragment conjugated with KLH as a synthetic antigen (Figure 4).
 Figure 4: Method for producing antibodies that specifically recognize ciguatoxin using synthetic haptens.
Figure 4: Method for producing antibodies that specifically recognize ciguatoxin using synthetic haptens.
Antibody10C9 bound very strongly to the ABCDE fragment as well as ciguatoxin CTX3C. It showed no binding to other polyether marine toxins such as brevetoxin, okadaic acid, and mitotoxin (Figures 5).8)
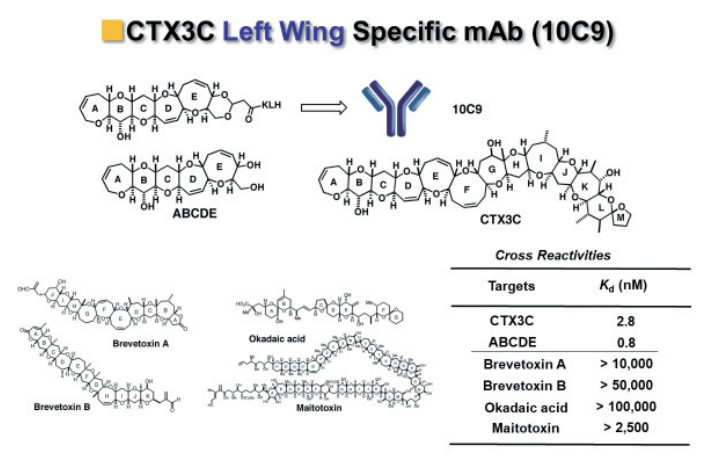 Figure 5: Binding specificity of anti-CTX3C monoclonal antibody 10C9
Figure 5: Binding specificity of anti-CTX3C monoclonal antibody 10C9
Similarly, we succeeded in producing specific monoclonal antibodies 3D11 and 8H4 that bound to the right side of CTX3C and CTX1B, respectively (Figure 6).8, 9)
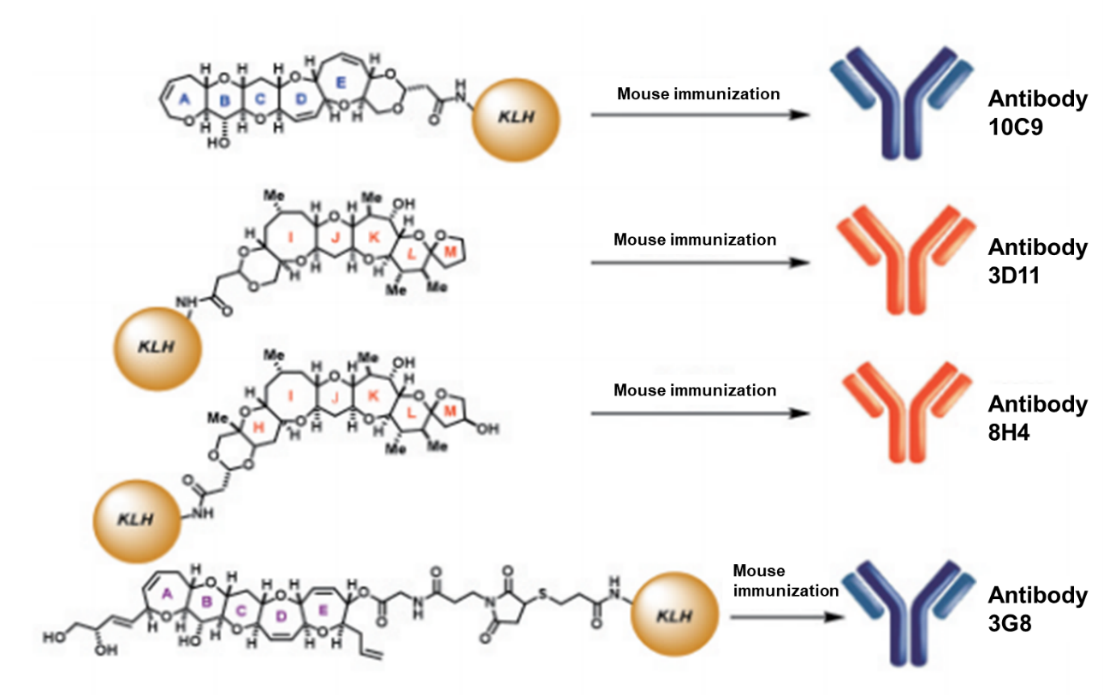 Figure 6: Four anti-ciguatoxin monoclonal antibodies obtained by immunization of synthetic haptens conjugated with KLH
Figure 6: Four anti-ciguatoxin monoclonal antibodies obtained by immunization of synthetic haptens conjugated with KLH
On the other hand, it took more time to produce antibody which bound to the left side of CTX1B4, 5), the most common causative toxin of CFP in Okinawa and other Pacific waters. We prepared ABCD and ABCDE fragment-KLH conjugates in a similar manner to 10C9, 3D11, and 8H4. These conjugates were immunized in mice by Drs. Fujii and Tsumuraya, but we obtained no good results. Thus, they tried other methods, such as immunization with mice of autoimmune disease and the phage display method. However, they were not successful. After much discussion, we asked ourselves, "As long as a synthetic hapten-KLH conjugate has been synthesized correctly, the antibody could be produced when immunized in mice." Something must be wrong in the case of the left half of CTX1B. We looked into the structural difference between the left halves of CTX1B and CTX3C. Apparently, the big structural difference is the presence of a dihydroxybutenyl substituent on the A ring of CTX1B. We suspected that its primary hydroxyl group might cause a trouble, and hypothesized that an active ester of the ABCD- or ABCDE-ring model of CTX1B might react with another active esters before and/or after binding to lysine residue of the protein due to its high nucleophilicity. Oligomeric haptens might be formed, resulting in insufficient amount of haptens on the surface of the protein. (Figure 7).
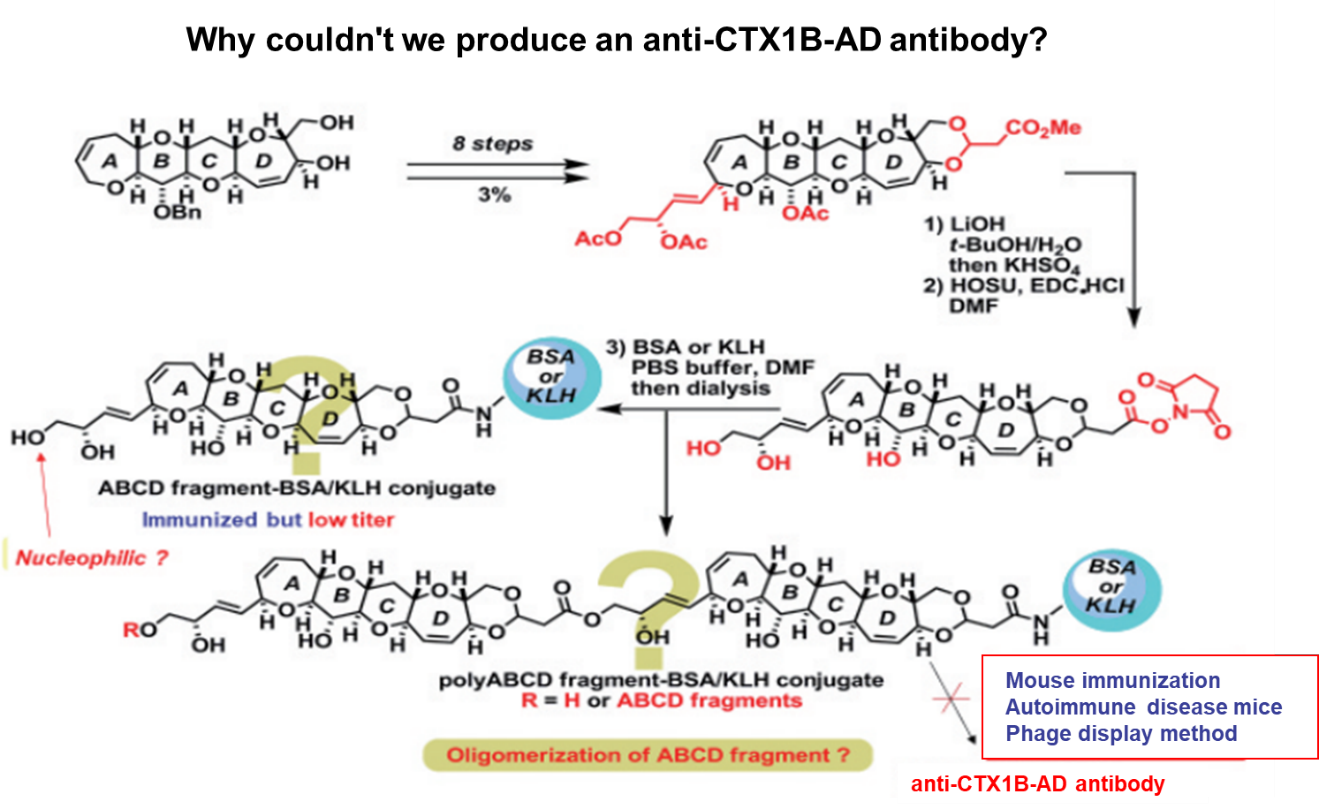 Figure 7: (hypotheses) Reasons why conventional methods cannot produce effective antigens.
Figure 7: (hypotheses) Reasons why conventional methods cannot produce effective antigens.
Therefore, we turned our attention to the thiol strategy which might use more nucleophilic thiol and Michael acceptor to conjugate the hapten with the protein. At first, we attached a thiol to the hapten and a Michael acceptor maleimide to the lysine residue without much success. Maleimide was then attached to the hapten, and protein was modified by thiol as shown in Figure 8. This strategy worked very well. Dr. Tsumuraya immunized mice with thus obtained hapten KLH conjugate antigen to give antibody 3G8 finally, which recognized the left side of CTX1B with high affinity and specificity.10,11)
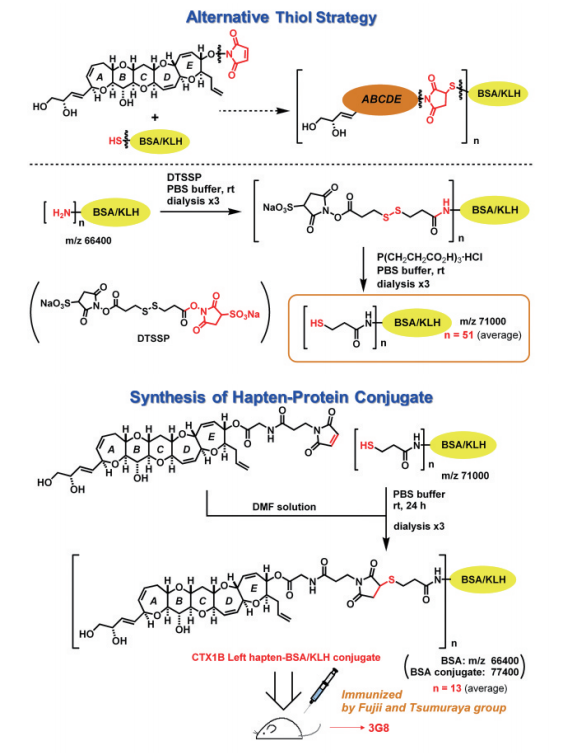 Figure 8: Thiol-based conjugate production method and anti-CTX1B monoclonal antibody 3G8
Figure 8: Thiol-based conjugate production method and anti-CTX1B monoclonal antibody 3G8
Development of Highly Sensitive Sandwich ELISA
Four anti-ciguatoxin antibodies are in our hand, Dr. Tsumuraya established a sandwich ELISA method using horse radish peroxidase (HRP) to detect ciguatoxins specifically (Figure 9).8-11)
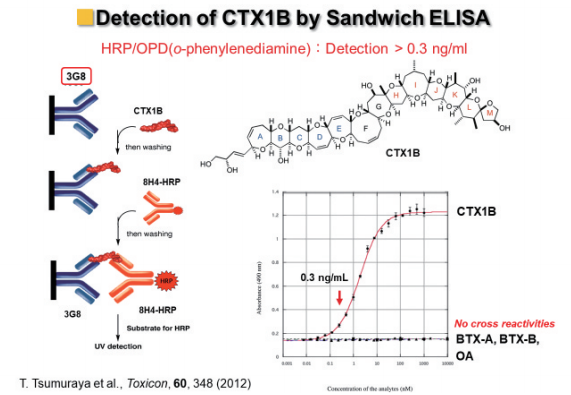 Figure 9: Sandwich ELISA using HRP
Figure 9: Sandwich ELISA using HRP
However, its sensitivity was not high enough for practical use. For example, FDA (US) issued a guidance level of 0.01 ppb CTX1B equivalent toxicity in edible fish.12) Since 1997, our research was financially supported by JST-CREST program and the total synthesis of ciguatoxins and collaborative studies with Drs. Fujii and Tsumuraya were greatly accelerated as shown above. In 2002, CREST finished and followed by JST-SORST. In this occasion, another collaborator Takeshi Sato, President of Cell Science Inc. Sendai, joined our research, who was also introduced by Prof. Ishida. Pres. Sato quickly started and dramatically improved the sensitivity of ELISA on CTX3C by using fluorescence and chemiluminescence. Furthermore, he built a prototype of a simple and quick detection kit using colloidal gold and its application to detect ciguatoxin in blood.
Subsequently, Dr. Tsumuraya re-examined the ALP-fluorescence sandwich ELISA using the four antibodies and improved the highly sensitive detection of the major Pacific ciguatoxins, CTX3C, 51-hydroxy-CTX3C, CTX1B, and 54-deoxy-CTX1B (Figures 10, 11).
Finally, he demonstrated that our ALP-fluorescent sandwich ELISA can detect ciguatoxins in fish, and furthermore he established the sandwich ELISA can detect the four ciguatoxins at once using 10C9, 3G8 and 8H4-ALP (Figure 12).12, 13)
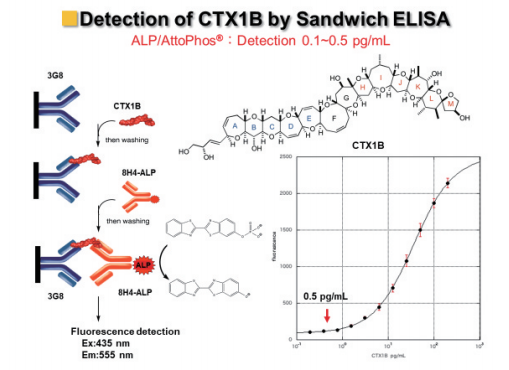 Figure 10: Highly sensitive sandwich fluorescence ELISA using ALP.
Figure 10: Highly sensitive sandwich fluorescence ELISA using ALP.
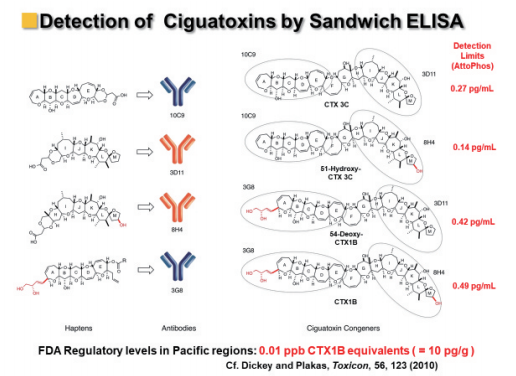 Figure 11: Highly sensitive sandwich fluorescent ELISA for detection of four ciguatoxins.
Figure 11: Highly sensitive sandwich fluorescent ELISA for detection of four ciguatoxins.
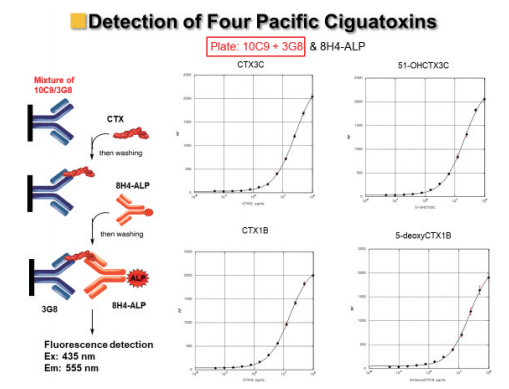 Figure 12: Highly sensitive sandwich fluorescent ELISA for detection of four ciguatoxins at once.
Figure 12: Highly sensitive sandwich fluorescent ELISA for detection of four ciguatoxins at once.
Pres. Sato improved the ELISA kit of CTX1B and finally produced the CTX-ELISA 1B as the first kit for practical use. This kit has been marketed by Fujifilm Wako. Thus, my dream of 30 years came true.
Summary
I have looked back herein our 30-year studies: synthetic studies of ciguatoxins, production of anti-ciguatoxin monoclonal antibodies by Dr. Tsumuraya using pentacyclic synthetic intermediates-KLH conjugates as antigens, development of sandwich ELISA, establishment of a practical ELISA CTX1B kit by Pres. Sato of Cell Science Ltd., and marketing by Fujifilm Wako. I would like to express my deepest gratitude to all those who cooperated with me.
I hope the ELISA kit to detect CTX3C will soon be realized and the simple kit developed by Pres. Sato will also be marketed. We also confirmed that our monoclonal antibodies can be used to detoxify (treat) mice poisoned with ciguatoxin.14) Genetic engineering of mouse anti-ciguatoxin monoclonal antibodies into humanized monoclonal antibodies is also in progress by Dr. Tsumuraya and Dr. Fujii. I dream further that these ciguatoxin detection kits will be used worldwide by researchers, public health officials, and fishermen to prevent ciguatera poisoning, and moreover, to help treat patients of ciguatera poisoning.
I emphasize that the total synthesis can play an important role for basic and applied sciences and technologies. I would like to thank again all the collaborators and students who worked on total synthesis and artificial antigen synthesis in Tohoku University.
Reference
- Yasumoto, T. and Murata, M.: Chem. Rev., 93, 1897 (1993). DOI: 10.1021/cr00021a011
- Lewis, R. J.: Toxicon, 39, 97 (2001). DOI: 10.1016/S0041-0101(00)00161-6
- Dickey, R. W. and Plakas, S. M.: Toxicon, 56, 123 (2010). DOI: 10.1016/j.toxicon.2009.09.008
- (a) Murata, M., Legrand, A. M., Ishibashi, Y. and Yasumoto, T.: J. Am. Chem. Soc., 111, 8929 (1989). DOI: 10.1021/ja00206a032
(b) Murata, M., Legrand, A.-M., Ishibashi, Y., Fukui, M. and Yasumoto, T.: J. Am. Chem. Soc., 112, 4380 (1990). DOI: 10.1021/ja00167a040
(c) Satake, M., Fukui, M., Legrand, A.-M., Cruchet, P. and Yasumoto, T.: Tetrahedron Lett., 39, 1197 (1998). DOI: 10.1016/S0040-4039(97)10808-5
(d) Yasumoto, T., Igarashi, T., Legrand, A.-M., Cruchet, P., Cinain, M., Fujita, T. and Naoki, H.: J. Am. Chem. Soc., 122, 4988 (2000). DOI: 10.1021/ja9944204 - (a) Yogi, K., Oshiro, N., Inafuku, Y., Hirama, M. and Yasumoto, T.: Anal. Chem., 83, 8886 (2011). DOI: 10.1021/ac200799j
(b) Otero, P., Pérez, S., Alfonso, A., Vale, C., Rodríguez, P., Gouveia, N. N., Gouveia, N., Delgado, J., Vale, P., Hirama, M., Ishihara, Y., Molgó, J. and Botana, L. M. : Anal. Chem., 82, 6032 (2010). DOI: 10.1021/ac100516q - (a) Oguri, H., Tanaka, S., Hishiyama, S., Oishi, T., Hirama, M., Tsumuraya, T., Tomioka, Y. and Mizugaki, M.: Synthesis (Special Issue), 1431 (1999). DOI: 10.1055/s-1999-3646
(b) Nagumo, Y., Oguri, H., Shindo, Y., Sasaki, S., Oishi, T., Hirama, M., Tomioka, Y., Mizugaki, M. and Tsumuraya, T.: Bioorg. Med. Chem. Lett. 11, 2037 (2001). DOI: 10.1016/s0960-894x(01)00358-4
(c) Nagumo, Y., Oguri, H., Tsumoto, K., Shindo, Y., Hirama, M., Tsumuraya, T., Fujii, I., Tomioka, Y., Mizugaki, M. and Kumagai, I.: J. Immunol. Methods, 289, 137 (2004). DOI: 10.1016/j.jim.2004.04.003 - (a) Hirama, M., Oishi, T., Uehara, H., Inoue, M., Maruyama, M., Oguri, H. and Satake, M.: Science, 294, 1904 (2001). DOI: 10.1126/science.1065757
(b) Inoue, M., Miyazaki, K., Uehara, H., Maruyama, M. and Hirama, M.: Proc. Natl. Acad. Sci. U. S. A., 101, 12013 (2004). DOI: 10.1073/pnas.0401684101
(c) Inoue, M., Miyazaki, K., Ishihara, Y., Tatami, A., Ohnuma, Y., Kawada, Y., Komano, K., Yamashita, S., Lee, N. and Hirama, M.: J. Am. Chem. Soc., 128, 9352 (2006). DOI: 10.1021/ja063041p
(d) Yamashita, S., Ishihara, Y., Morita, H., Uchiyama, J., Takeuchi, K., Inoue, M. and Hirama, M.: J. Nat. Prod. 74, 357 (2011). DOI: 10.1021/np100729d
(e) Yamashita, S., Takeuchi, K., Koyama, T., Inoue, M., Hayashi, Y. and Hirama, M.: Chem. Eur. J., 21, 2621 (2015). DOI: 10.1002/chem.201405629
(f) Inoue, M. and Hirama, M.: Acc. Chem. Res., 37, 961 (2004). DOI: 10.1021/ar0301577
(g) Inoue, M. and Hirama, M.: Synlett, 577 (2004). DOI: 10.1055/s-2004-817767
(h) Hirama, M.: Chem. Rec., 5, 240 (2005). DOI: 10.1002/tcr.20049 - (a) Oguri, H., Hirama, M., Tsumuraya, T., Fujii, I., Maruyama, M., Uehara, H. and Nagumo, Y.: J. Am. Chem. Soc., 125, 7608 (2003). DOI: 10.1021/ja034990a
(b) Tsumuraya, T., Fujii, I. and Hirama, M.: Toxicon, 56, 797 (2010). DOI: 10.1016/j.toxicon.2009.06.003 - Tsumuraya, T., Fujii, I., Inoue, M., Tatami, A., Miyazaki, K. and Hirama, M.: Toxicon, 48, 287 (2006). DOI: 10.1016/j.toxicon.2006.05.014
- Tsumuraya, T., Takeuchi, K., Yamashita, S., Fujii, I. and Hirama, M.: Toxicon, 60, 348 (2012). DOI: 10.1016/j.toxicon.2012.04.347
- Tsumuraya, T., Fujii, I. and Hirama, M.: J. AOAC Int., 97, 373 (2014). DOI: 10.5740/jaoacint.SGETsumuraya
- Tsumuraya, T., Sato, T., Hirama, M. and Fujii, I.: Anal. Chem., 90, 7318 (2018). DOI: 10.1021/acs.analchem.8b00519
- Tsumuraya, T. and Hirama, M.: Toxins, 11, 533 (2019). DOI: 10.3390/toxins11090533
- Inoue, M., Lee, N., Tsumuraya, T., Fujii, I. and Hirama, M.: Toxicon, 53, 802 (2009). DOI: 10.1016/j.toxicon.2009.02.017




