Chiral Amino Acid Analysis Using LC/MS
This article was written by Sachise Karakawa, Research Institute for Bioscience Products & Fine Chemicals, Ajinomoto Co., Inc., for Vol. 92, No. 1 (January 2023) of Wako Junyaku Jiho.
Introduction
Amino acids are the building blocks of proteins, and have various physiological functions related to neurotransmission, immunity, etc. Apart from glycine, amino acids have optical isomers (isomers with identical physical and chemical properties other than optical rotation), which are designated as D- and L-amino acids. Protein-constituent amino acids and many amino acids present in living organisms are almost all L-amino acids, but trace amounts of D-amino acids also exist and are known to have physiological functions different from those of L-amino acids. Representative D-amino acid functions include the role of D-serine in neurotransmission, learning, and memory functions in the brain, and the role of D-aspartic acid in the regulation of hormone synthesis and secretion. It has also been reported that various D-amino acids, including D-alanine, D-glutamic acid, and D-aspartic acid, are produced and used as peptidoglycan components in bacteria and that D-alanine plays a significant role in regulating osmotic pressure in aquatic invertebrates such as shrimp and bivalves. It is also known that commonly used fermented foods such as alcoholic beverages, vinegar, and lactic acid beverages contain more D-alanine, D-glutamic acid, and D-aspartic acid than other beverages. In recent years, the availability of D-amino acids as biomarkers has also been reported, since the plasma levels of D-amino acids fluctuate in patients with chronic kidney disease and mild cognitive impairment (MCI), and it is anticipated that they will be utilized in diagnosis and drug discovery. However, in general, compared to L-amino acids, the existence and functions of D-amino acids are still unclear in many cases, attracting a high level of interest from researchers.
Analysis of chiral amino acids
In general, the amounts of D-amino acids in living organisms is very low compared to L-amino acids, making them very difficult to detect and quantify, and the lack of serviceable analytical technology has been a bottleneck in D-amino acid research. Therefore, to date, only analytical researchers and their collaborators who have developed new D-amino acid analytical methods have made progress in clarifying the existence, distribution, variation, and function of D-amino acids. In other words, optimal analytical methods for D-amino acids are needed to support D-amino acid research.
In order to analyze trace amounts of D-amino acids in complex samples such as biological and food samples, high detection sensitivity and high selectivity that enables separation from other impurities including L-amino acids are required. There are two approaches to the separation of D- and L-amino acids: the direct method and the indirect method.1) The direct method is a technique in which D- and L-amino acids are separated in a chiral column and measured by a fluorescence detector or mass spectrometer (MS). Representative techniques developed by Prof. Hamase et al. (Kyushu University, Japan) include a two-dimensional HPLC method combining a reverse phase column and a chiral column followed by fluorescent derivatization using 4-fluoro-7-nitro-2,1,3-benzoxadiazole (NBD-F).2) In the indirect method, D- and L-amino acids are reacted with a chiral derivatization reagent to form diastereomers, which are separated by a reverse phase column and then measured by a fluorescence detector or MS. A typical method is diastereomer derivatization of amino acids using o-phthalaldehyde (OPA) and chiral thiols, separation in a reverse phase column, followed by measurement using a fluorescence detector.3) Since amino acids are zwitterionic substances and are highly hydrophilic, they are poorly retained in reverse phase columns. Derivatization confers hydrophobicity, which is generally used to enhance retention and separation in reverse phase columns. Derivatization of amino groups can selectively detect only compounds with amino groups, and improve separation and sensitivity by introducing a chiral backbone with fluorescent molecules or molecules that are easily ionized by MS as the detection group of the derivatization reagent. Therefore, a variety of derivatization reagents have been developed for amino acid analysis.
Amino acids analysis using LC/MS
In recent years, liquid chromatography-mass spectrometry (LC/MS) has been increasingly used for amino acid analysis of biological and food samples. The use of a mass spectrometer (MS) for detection allows analytes to be detected by mass, making analysis more selective than conventional UV or fluorescence detection methods. Conventional amino acid analysis methods using high-performance liquid chromatography (HPLC) and post-column derivatization with a ninhydrin reagent can analyze about 40 amino acids in about 120 minutes with a high degree of accuracy and simplicity, while amino acid analysis using LC/MS is fast and highly sensitive. A typical example of LC/MS amino acid analysis is a method using pre-column derivatization with Fujifilm Wako's reagent APDSTAG®. This method enables analysis of 38 amino acids in a very short time of 8 minutes.4) In addition, the "UF-Amino Station" has been developed and marketed by Shimadzu Co., Inc. as an analytical instrument that performs APDSTAG® derivatization automatically, and is used in biomarker research, amino acid product development, and other research requiring amino acid analysis of multiple samples. On the other hand, it is important to note that the detection sensitivity of MS can vary greatly due to ionization suppression or ionization enhancement caused by matrix effects. Since the matrix effect varies markedly depending on the sample and analyte, it is necessary to correct the result by using stable isotopes that elute at the same retention time as an internal standard in order to perform precise quantitative analysis. "APDSTAG® Wako Amino Acids Internal Standard Mixture Solution" (mixture solutions of stable isotopes of amino acids), which are essential for the precise quantification of amino acids by LC/MS, are available from the Fujifilm Wako.
(R)-BiAC pre-column derivatization for LC/MS chiral amino acid analysis ((R)-BiAC method)
Analysis using APDSTAG® allows quantification of each amino acid, such as alanine, serine, and glutamic acid, but because L-alanine and D-alanine cannot be separated, they are quantified as the total combined amount of l and D-alanine. In many amino acid studies, this unseparated data are interpreted as almost exclusively L-alanine. However, some samples may contain trace amounts of D-alanine that must be taken into account. Therefore, it is necessary to analyze D- and L-alanine separately in order to rigorously evaluate amino acid functions and variations.
As a method for analyzing chiral amino acids by LC/MS, we have independently developed an axially chiral derivatization reagent ((R)-BiAC (Biaryl axially chiral-tag)) and established a method for simultaneous analysis of D and L-amino acids by LC/MS.5) The (R)-BiAC reagent is designed to selectively label amino acids by reacting with amino groups to form diastereomers, enabling separation of D- and L-amino acids on a reverse phase column and highly sensitive detection by MS (Figure 1).
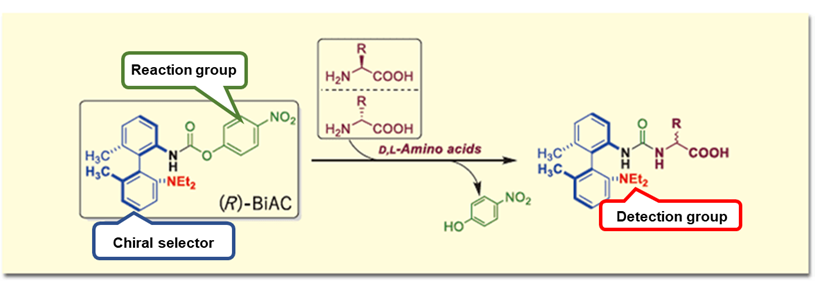
Figure 1. (R)-BiAC structure and reaction with D, L-amino acids
In the (R)-BiAC method, by combining the derivatization reagent structure of the axial chiral skeleton with the separation performance using a high resolution sub-2 μm column, 19 D- and L-amino acids can be simultaneously analyzed with a resolution (Rs) 1.9 or better and an analysis time of 19 minutes. Furthermore, the excellent MS sensitivity of the (R)-BiAC detection group enables detection at the attomolar level per injection, a very high sensitivity not achievable with existing reagents, and enables analysis of trace amounts of D-amino acids with high reproducibility. The extraction MS chromatogram of a standard solution of D, L-amino acids is shown in Figure 2.
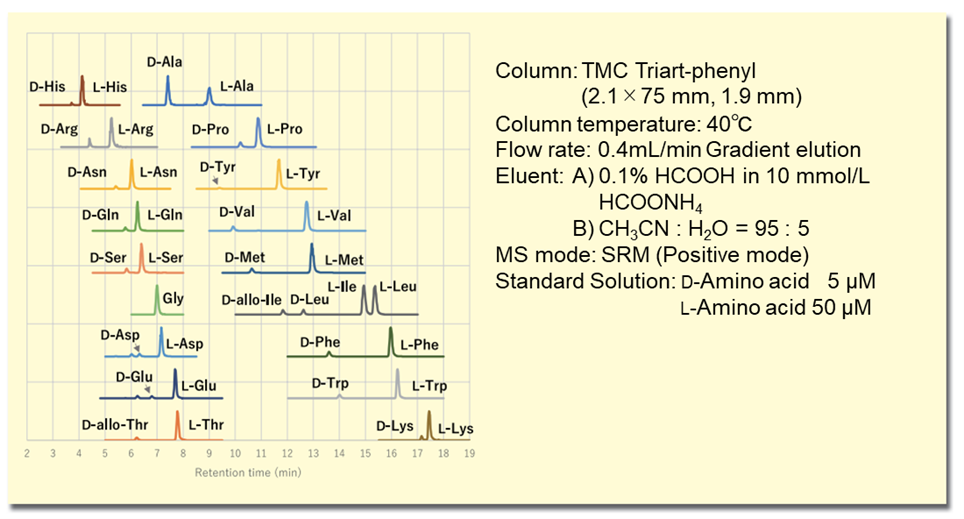
Figure 2. MS chromatogram of amino acid standard solution derivatized with (R)-BiAC
The (R)-BiAC method is characterized by the fact that the D-amino acid elutes before the L-amino acid. In samples with an excess of L-amino acid, such as biological samples, the D-amino acid elutes before the L-amino acid, which has the advantage that the D-amino acid is not obscured by the L-amino acid peak (Figure 3).
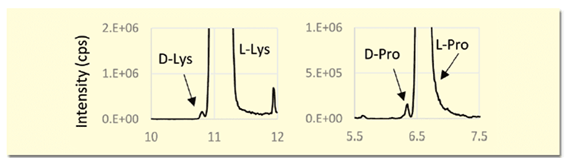
Figure 3. Detection of trace amounts of D-amino acids in biological samples (extraction MS chromatogram)
The (R)-BiAC method has good sensitivity and selectivity, can be applied to a wide range of samples including food, biological (plasma, urine, feces, tissue, cells), and microorganism samples. Furthermore, it is a versatile analytical method that can be performed by anyone with the correct reagents ((R)-BiAC) using a liquid chromatography-mass spectrometer.6, 7) Figure 4 shows an MS chromatogram of D, L-amino acids in a lactic acid beverage as an example of food analysis.
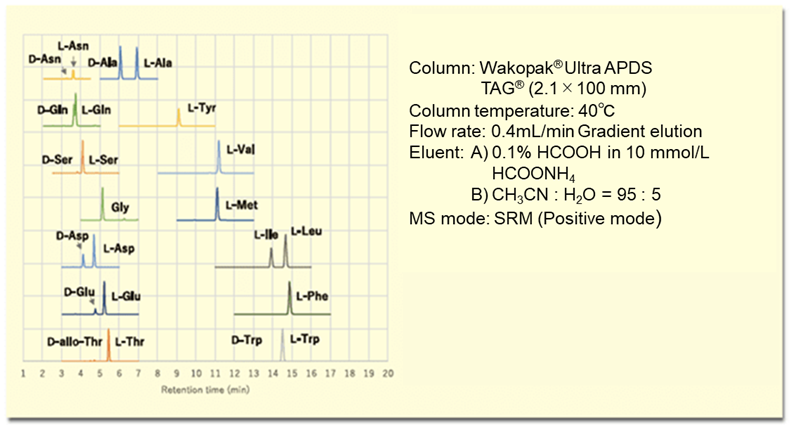
Figure 4. MS chromatogram of D, L-amino acids in a lactic acid beverage derivatized with (R)-BiAC.
From Figure 4, the presence of D-amino acids such as D-alanine, D-glutamic acid, and D-aspartic acid in a lactic acid beverage can be clearly confirmed. This (R)-BiAC reagent is available from Fujifilm Wako.
Conclusion
The ability to easily analyze D-amino acids has led to the clarification of their functions and usefulness in various fields. In addition to the analytical methods described in this paper, many other amino acid analytical methods using LC/MS and chiral amino acid analytical methods that separate D- and L-amino acids have been reported, but their characteristics and performance vary. In many methods, measurement is possible with standard solutions, but quantification cannot be performed with actual samples due to the influence of impurities or matrix effects. To prevent this from happening, it is necessary to validate the analytical method for the analyte in advance.
This (R)-BiAC reagent is expected to accelerate D-amino acid research because it provides the sufficient sensitivity and selectivity for any sample and makes validation of analytical methods easy.
References
- Karakawa, S. et al.: Wako Junyaku Jiho, 87 (1), 5 (2019).
- Ishii, C. et al.: Chromatography, 41, 1 (2020).
- Gogami, Y. et al.: Chromatogr. B, 879, 3259 (2011).
- Shimbo, K. et al.: Rapid Commun. Mass Spectrom. 23, 1483 (2009).
- Harada, M. et al.: Chromatogr. A, 1593, 91 (2019).
- Harada, M. et al.: Symmetry, 12, 913 (2020).
- Karakawa, S. et al.: Chromatography, 44, 21 (2023).




