Precautions in Pre-treatment of Environmental Water Samples by Chelating Resin Preconcentration Method
The original article by Dr. Eiji Fujimori of the National Environmental Research and Training Institute, Ministry of the Environment, appeared in an e-newsletter of FUJIFILM Wako and was translated by the company.
Introduction
Inductively coupled plasma mass spectrometry (ICP-MS) is capable of simultaneous analysis of multiple elements at extremely low levels and is the most sensitive elemental analysis method among common analytical methods. In the field of environmental analysis, the scope of application is rapidly expanding with the development of collision/reaction cell technology.
A solid-phase extraction method using iminodiacetic acid-type chelating resin (chelating resin preconcentration method) has been widely used as a pretreatment method for environmental water samples for ICP-MS measurements, because it can simultaneously achieve multi-element concentration of transition metals and removal of alkali and alkaline earth metals. The chelating resin preconcentration method was adopted as the official analytical method, "Testing Methods for Industrial Wastewater" in the 2013 revision of JIS K0102, and its application is expected to expand further in the future.
There are, however, various issues to address when applying the chelating resin preconcentration method to the analysis of environmental waters. Based on the author's experience, this article describes sample pretreatment, countermeasures against spectral interferences in ICP-MS, and precautions in the preparation of standard solutions.
Influence of Coexisting Chelating Agents When Applying Chelating Resin Preconcentration Method to Environmental Waters
As mentioned above, the chelating resin preconcentration method is capable of simultaneous concentration of multiple transition metals. Many consider that the target elements can be recovered almost quantitatively if the pH during the adsorption is adjusted to 4.0 to 6.0. However, when the author applied the chelating resin preconcentration method to river water samples containing sewage effluent, a lower recovery rate was observed for several elements. After examining various possibilities, the current thought is that chelating agents such as ethylenediaminetetraacetic acid (EDTA) in wastewater could cause the decreased recovery rate 1).
Figure 1 summarizes the recovery rates when a mixture of Fe, Co, Ni, Cu, Zn, Cd, and Pb (10 μg/L each) was concentrated using Presep® PolyChelate (hereafter, "Presep®") in the presence of various concentrations of EDTA. Note that Presep® resin was taken from a prefilled 3-mL syringe (at top in photo) and filled into an empty 12-mL reservoir (photo, bottom) to improve ease of use.
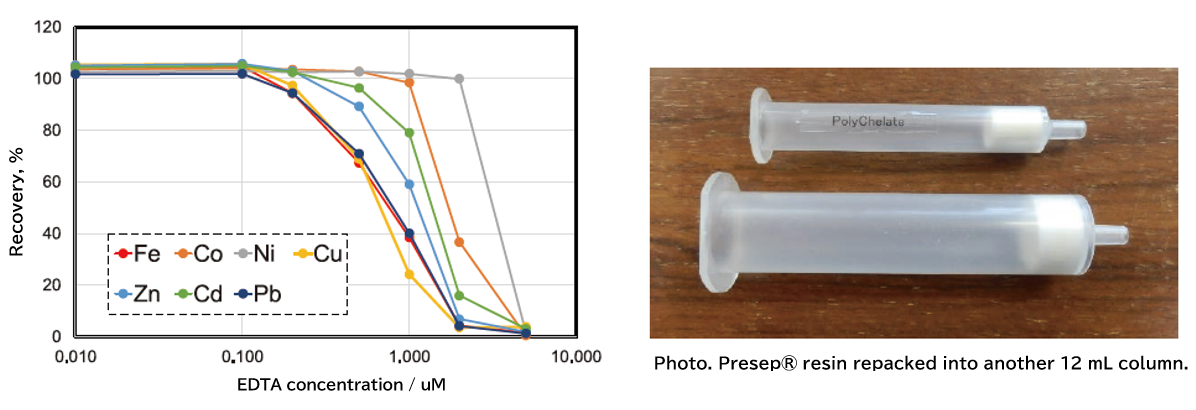
Fig. 1. The recovery rate change of metals by coexisting EDTA concentration.
As shown in Figure 1, a decrease in the recovery of each element was observed when the concentration of coexisting EDTA exceeded 0.1 μM, and the recovery of all elements was less than 4% when 5 μM of EDTA was present. If reduced recovery due to coexisting chelating agents is suspected, a high-temperature acid treatment of the sample could be effective.
After testing various conditions, we showed that effects of coexisting EDTA and other substances can be eliminated by adding 2.5 mL of nitric acid and 1 mL of hydrogen peroxide to 50 mL of sample and heating at 170°C or higher for 4 hours 1). It is necessary to conduct a spike recovery test when applying the chelating resin preconcentration method to industrial and domestic wastewater containing chelating agents such as EDTA, as well as river water and coastal seawater that receive such wastewater. An appropriate pretreatment (such as acid treatment) is required if a decrease in recovery rate is observed.
Spectral Interference from Molybdenum in Cadmium Measurement
One drawback of the iminodiacetic acid chelating resin preconcentration method is the poor recovery of Mo and other elements that form oxoacid anions at pH 4.0 to 6.0, a range that is generally suitable for the recovery of transition metals. To avoid this drawback, various improved iminodiacetic acid-type chelating resins have become commercially available. One of them is Presep®, which provides a great advantage in that transition metals and Mo can be recovered at the same pH.
Figure 2 shows a comparison of Presep® versus several commercially available solid-phase extraction columns (Hitachi High-Tech NOBIAS PA1 [NOBIAS], G.L. Science InertSEP® ME-2 [ME-2], and InertSEP® ME-1 [ME-1]) for Cd and Mo recovery at pH 5.0. As evident from Figure 2, Cd and Mo can be quantitatively recovered at the same pH (pH 5.0) using Presep®.
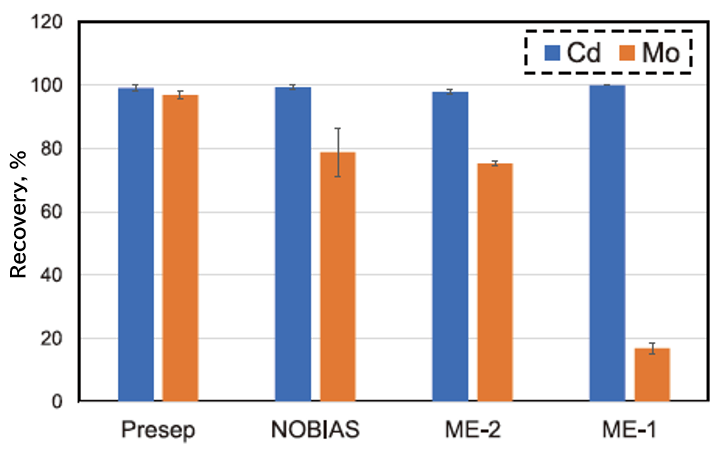
Fig. 2. Difference in recovery rate of Cd and Mo by solid-phase extraction column.
This feature, however, can be problematic for ICP-MS analysis of Cd at ultra-trace levels. In the measurement of Cd by ICP-MS, polyatomic ion interferences by Mo oxide ions (95Mo16O → 111Cd, 98Mo16O → 114Cd) are known. In cases of seawater samples, which contain about 10 μg/L of Mo, interference caused by MoO is not negligible when measuring extremely low levels (ng/L level) of Cd. In such cases, mathematical correction for polyatomic ion interference becomes necessary 2).
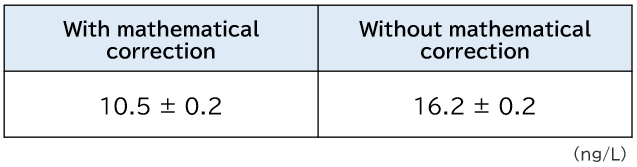
As an example, below is the result of Cd measurements by ICP-MS after coastal seawater was preconcentrated 40-fold using Presep®. This coastal seawater sample contained about 10 μg/L of Mo, and when preconcentrated 40-fold, the concentration in the eluate (used for the measurement) was about 300 μg/L. Table 1 shows the result of Cd measurements when the coastal seawater sample was preconcentrated 40-fold with Presep®. Cd concentration was 16.2 ng/L without MoO interference correction, while it was 10.5 ng/L with correction. The difference, 5.7 ng/L, was due to the concentration that stems from the polyatomic ion interference from MoO.
Table 1.Difference in Cd measurement results in coastal seawater with and without mathematical correction
In the case of open ocean seawater with Cd concentrations at the 1 ng/L level, mathematical correction is difficult to apply, and selective removal of Mo by chemical separation is necessary. See the references 2, 3) for selective Mo removal methods.
Precautions in the Preparation of Multi-Element Standard Solutions for ICP-MS Measurements
When preparing multi-element standard solutions for ICP-MS measurement, the following precautions should be taken: (1) avoid mixing elements that may cause spectral interference (e.g., Mo and Cd), (2) avoid mixing elements that may cause precipitation (e.g., Ag and Cl), (3) avoid mixing elements with widely different concentrations (e.g. mg/L and μg/L), and (4) for elements that are stable in the absence of nitric acid, dilutions with nitric acid solution should be made just before measurement. Below is an example of rare earth elements (in this case, lanthanides from La to Lu) to elucidate point (1) above.
When using ICP-MS for the measurement of rare earth elements, caution should be made to avoid spectral interference among them (isobaric ions, oxide ions, and hydroxide ions). When measuring rare earth elements, masses (m/z) that are free of isobaric interferences should be selected. In addition, polyatomic ion interferences from oxide and hydroxide ions must be subjected to mathematical correction.
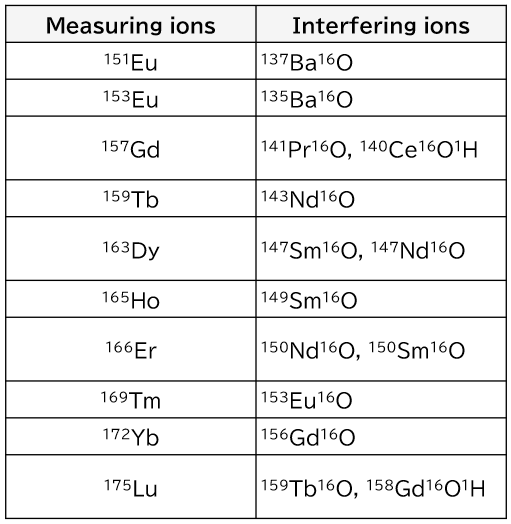
The main polyatomic ion interferences that are problematic when measuring rare earth elements by ICP-MS are listed in Table 2. If problematic polyatomic ion interferences are known in advance, mixing of those elements should be avoided.
Table 2. Major problematic polyatomic ion interferences when measuring rare earth elements
For instance, the author mixes the standard solutions for calibration curves in four groups: Group I (La, Ce, Lu), Group II (Pr, Nd), Group III (Sm, Eu, Gd, Tb), and Group IV (Dy, Ho, Er, Tm, Yb). For rare earth elements and other elements, multi-element mixtures for ICP-AES and ICP-MS are commercially available. It is important to take polyatomic ion interference into consideration and decide on combinations as needed.
Conclusions
To demonstrate the points in this article in summary, below are the results of the analysis of rare earth elements in water from the Tama River as an example of the application of chelating resin preconcentration and ICP-MS measurement. Rare earth elements can be used as indicators of the cycling and distribution of elements in the environment, and their precise analysis is essential.
Since a large amount of sewage effluent flows into the Tama River, there was a concern that coexisting chelating agents such as EDTA may reduce the recovery rate. Therefore, 10 mL of nitric acid and 4 mL of hydrogen peroxide were added to 200 mL of each sample and heated at 200°C for 4 hours. After the degradation, samples were preconcentrated 40-fold using Presep®. Standard solutions prepared in four groups as mentioned above were used in the ICP-MS measurements, and mathematical correction for spectral interferences was applied to obtain the measured values.
Table 3 shows the results of the measurements of rare earth elements in the river water at the lowest reaches of the Tama River. As can be seen from the table, it was possible to accurately measure rare earth elements at extremely low levels of ng/L in the samples.
Table 3. Analysis results for rare earth elements in downstream water from the Tama River obtained by chelating resin preconcentration/ICP-MS
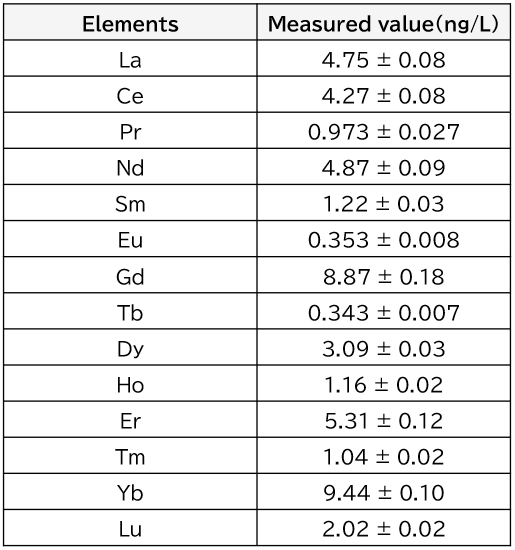
Figure 3 shows the so-called rare earth element distribution pattern, which is a plot of the concentration of rare earth elements in a sample normalized by a reference material (in this case, shale) in order of atomic number. Without going into details, these patterns are used when the characteristics of the distribution of rare earth elements are discussed. The water of the Tama River is characterized by a large positive anomaly of Gd and a larger rightward trend in the plot in the downstream water 1).
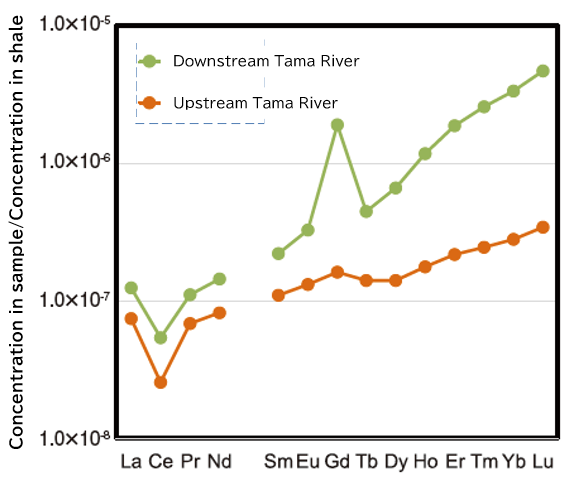
Fig. 3. Rare earth element distribution pattern in Tama River water
The chelating resin preconcentration method is a very useful pretreatment method for the precise analysis of elements at the ng/L level in environmental waters using ICP-MS. It has, however, various pitfalls as described in this article. The information presented here is only a part of what has become clear based on the author's actual experience. Problems and their countermeasures will differ depending on the sample and measurement device, and readers are advised to take necessary precautions.
References
- E. Fujimori et al. : Chemosphere, 214, 288 (2019).
- E. Fujimori : BUNSEKI KAGAKU (The journal of the Society for Analytical Chemistry), 65, 275 (2016). (in Japanese)
- Y. Takaku et al. : BUNSEKI KAGAKU(The journal of the Society for Analytical Chemistry), 65, 399 (2016). (in Japanese)
Related Products
SPE Columns for Metal (Elemental) Capture and Recovery "Presep® PolyChelate"




