Role of BDNF during development and its relation to neurodevelopmental disorders
This article was written by Dr. Shingo Suzuki, Department of Anatomy and Neurobiology, Faculty of Medicine, Kagawa University.
Introduction
Brain-derived neurotrophic factor (BDNF) is a trophic factor released mainly from neurons in a neural activity-dependent manner. It is involved in memory and learning by inducing synaptic organization/reorganization in the adult brain. Moreover, BDNF has a variety of functions in the developing brain, such as induction of neuronal survival, differentiation, synapse formation and synaptic connections. This range of functions makes it difficult to identify and understand its primary role in the developing brain, although its importance is well understood. Recent studies have suggested that decreased expression of BDNF during development may be associated with abnormalities in neural circuit formation, and these abnormalities may lead to developmental disorders. In this article, we describe the expression, function, and role of BDNF in the developing brain, and then explain the relationship between BDNF and neurodevelopmental disorders.
BDNF expression in the developing brain
BDNF is expressed in a wide range of brain regions, particularly in the hippocampus, amygdala, cerebrum, and cerebellum1, 2). BDNF is mainly expressed in excitatory neurons in the cerebral cortex and hippocampus. Expression of BDNF can be detected in the brain of mice at embryonic day 11.5, and is dramatically elevated during postnatal development3-5).
Since BDNF expression is affected by the neurotransmitters glutamate and GABA, as well as intracellular calcium signaling resulting from depolarization, the combination of these neurochemical conditions and developmental changes in the expression of transcription factors may result in changes in the level of BDNF expression. Interestingly, while expression of BDNF is elevated during development, the expression of TrkB, a high-affinity receptor for BDNF, does not change during this period.
It is widely known that BDNF expression is suppressed by DNA methylation6). There are numerous reports suggesting a link between decreased BDNF expression and DNA methylation that have been observed in animal models as well as in human pathology.
In addition, miRNAs and endogenous BDNF antisense RNAs have been shown to be factors that can suppress BDNF expression7, 8). Although BDNF is expressed as a number of transcripts that are differentially regulated, all mRNAs have the same coding region. In other words, only a protein with the same amino acid sequences is produced. Therefore, the diversity of BDNF transcripts is thought to contribute to the diversity of spatio-temporal expression in neurons9). For example, BDNF is released from neurons in both a constitutive and neural activity-dependent manner, but is generated as distinct transcripts.
BDNF is synthesized as a pro-form, which upon cleavage becomes mature BDNF10). The amino acid sequence corresponding to the region of mature BDNF is highly conserved and does not differ between human, mouse, and rat.
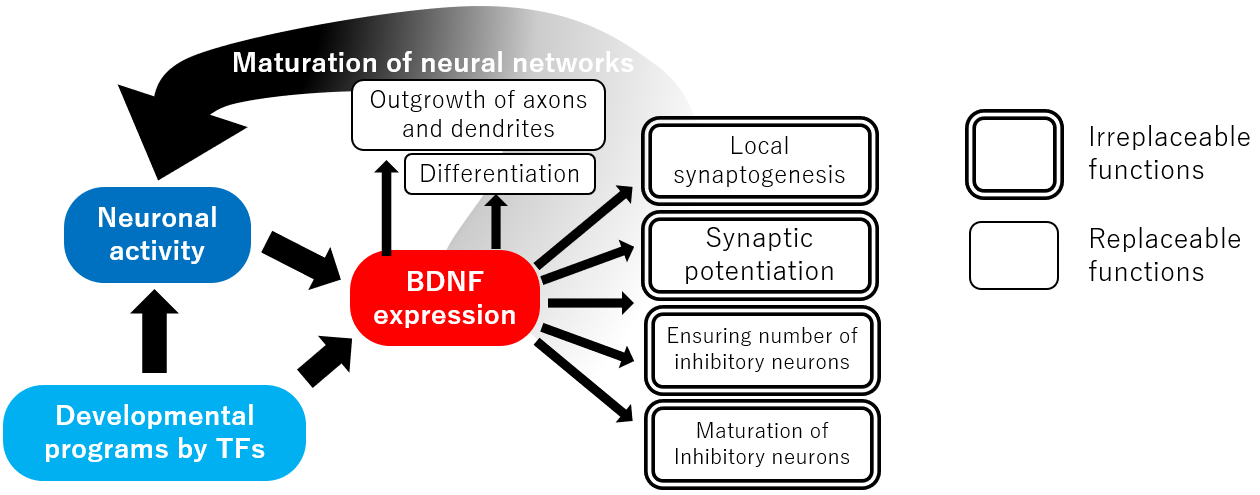 Figure. Functions of BDNF during development
Figure. Functions of BDNF during development
Function of BDNF in Central Neurons
BDNF acts on developing neurons in a number of ways. It can stimulate the proliferation of neural stem cells and their differentiation into neurons. BDNF also promotes neuronal survival, induces cell migration, and increases axon and dendrite branching and the number of dendrites11). Furthermore, BDNF can induce synaptic maturation, increase the number of synapses, increase the size of postsynaptic structures, regulate synaptic transmission, and promote long-term potentiation12). Interestingly, in some brain regions, BDNF has also been reported to induce axonal pruning13).
These diverse effects are phenomena observed in different parts of the brain at different times and in different experimental systems.
Key Role of BDNF during Development
Given the wide range of BDNF effects, it is reasonable to assume that a lack of BDNF would make it impossible for neural circuits to be created. Surprisingly, however, the brain structure of BDNF knockout mice is closer to normal than we expected. Of course, the effects of BDNF deficiency are significant, and most BDNF-knockout mice die within a few days of birth although some survive for 2-4 weeks14). However, these mice die due to the effects in tissues other than the brain, such as cardiac dysplasia. In their brains, the cortex and hippocampus, where BDNF is highly expressed in the wild-type mouse, no major changes such as partial loss in macroscale structure or disruption of the laminar structure are evident. Since BDNF is a protein that is expressed in increasing amount after birth, its specific function in brain development is likely to be exerted after the postnatal period when the neural circuitry arises and becomes functional. Indeed, BDNF homo-and hetero-zygous knockout mice or mice specifically deficient in neuronal BDNF show abnormalities in neural circuits and in synaptic functions15). These abnormalities are observed as learning and memory deficits and behavioral abnormalities. Therefore, the primary function of BDNF, which is difficult to replace, is its contribution to the formation of neural circuits and maintenance of brain function after birth. The neural circuits of the brain, which are created mainly during the fetal period, are completed after birth, reflecting environmental stimuli and experiences. BDNF is the most characteristic factor in this latter process and beyond.
One of the phenomena manifested by BDNF deficiency is a decrease in the number of inhibitory neurons in the hippocampus and cerebral cortex14). After cell migration, inhibitory neurons become quiescent and are incorporated into local circuits, where they are exposed to competition for survival. In contrast, in BDNF knockout mice, the number of excitatory neurons is unchanged; since BDNF is highly expressed in excitatory neurons, BDNF secreted by excitatory neurons stimulates the migration of inhibitory neurons, which then compete for survival, thereby promoting the growth of local inhibitory neurons. Since there are many factors that can induce neuronal survival, it is likely that the survival of inhibitory neurons during this period is particularly dependent on BDNF.
In addition, observations in the cortex and hippocampus in BDNF heterozygous and conditional knockout mice suggest that the effect of BDNF on branching in the peripheral axons and on synapse formation is greater than its effect on axonal extension and guidance16). Similarly, BDNF also affects the number of dendrites and branches, suggesting that BDNF actually increases the number of synapses by first laying the groundwork for increasing the number of synapses by promoting axonal and dendritic branching, and then inducing synapse formation. However, since BDNF is also known to contribute to synaptic and axonal pruning in the cerebellum and retina, the mechanism by which BDNF affects synapse formation in the brain is complex.
Studies using slices of postmature hippocampus have also reported that BDNF is selectively released from spines on single dendrites and can induce local synapse-specific long-term potentiation17) and that BDNF released from dendrites and cell bodies has the greatest effect on neurons in the vicinity18, 19). These findings suggest that BDNF may also function during development to increase the relative number of synapses in a localized area by being released locally.
BDNF is critically involved in the maturation of inhibitory neurons; in mice with forced expression of BDNF, the development of inhibitory neurons is accelerated and the termination of the critical period in the visual cortex, which depends on the development of inhibitory neurons, is brought forward5). Therefore, it is likely that the termination of the critical period is determined by the total amount of BDNF expressed over time. First, BDNF is released from neurons activated by incoming sensory stimuli. Then, BDNF expression is upregulated by the genetic development program and regulates the developmental period of inhibitory neurons.
In addition, prior to the critical period, immature electrophysiological properties and morphology of the parvalbumin-positive inhibitory neurons are observed in the barrel cortex of BDNF knockout mice20). This indicates that the effect of BDNF on the maturation of inhibitory neurons themselves is also important in the formation of local circuits at the subcritical stage. Maturation of neurons according to their timing and order is necessary for the correct formation of local circuits in the cortex21). Thus, it is quite possible that transient changes in BDNF expression during development may affect the structure of local circuits and influence brain function after maturation.
Furthermore, in the mature cerebrum, the parvalbumin-positive inhibitory neurons are connected to each other by gap junctions, leading to synchronous activity and rhythm generation. Therefore, it is possible that decreased BDNF expression during development may influence brain function by affecting synchronous firing as well as local circuit formation. Specific inhibition of BDNF signaling in parvalbumin-positive inhibitory neurons using genetically modified mice has been reported to affect synchronous activity and rhythm formation in the adult mouse22).
Although there is a long history of research into how BDNF is involved in synaptic potentiation within excitatory neural networks in the adult hippocampus23), the role of BDNF during development of excitatory neural networks is not fully understood. However, BDNF can positively affect the number and maturation of spines on the dendrites of excitatory neurons in the hippocampus and cerebral cortex, suggesting that BDNF may also influence the maturation of the excitatory neural networks. Since neural networks created by excitatory neurons are the mainstay of information processing systems and are also involved in the connection of inter-brain networks, it is highly desirable to elucidate the role of BDNF in the formation of these circuits.
BDNF and Neurodevelopmental Disorders
It is widely hypothesized that stress during development, including the fetal period, induces a decrease in BDNF expression, which in turn inhibits normal brain development and may predispose to the development of neurodevelopmental and psychiatric disorders. In support of this hypothesis, alterations in BDNF expression have been observed in animal models subjected to various stresses24). During the embryonic period, decreased BDNF expression and DNA methylation in the hippocampus have been observed in response to maternal restraint stress and psychological stress24). In addition, postnatal maternal separation also decreases BDNF expression in the hippocampus and prefrontal cortex, and a decrease in the number of synapses has been observed4).
When BDNF expression is decreased due to maternal separation stress, changes in the methylation status of histone and increased expression of miRNAs that target BDNF mRNA are observed24). Since animals exposed to stress in the fetal and neonatal stages show reduced sociality and anxiety-like behavior after development, it is possible that decreased BDNF expression may affect the formation of neural circuits during development. Considering the effects of BDNF during development, it is also possible that a stress-induced decrease in BDNF expression, even if transient and localized, may have a long-lasting effect on the individual.
On the other hand, a number of studies in humans have investigated the relationship between blood BDNF levels and psychiatric disorders. Blood BDNF is thought to be platelet-derived, but its significance is unknown. However, animal experiments have confirmed that group trends in the methylation of BDNF between blood and the brain are comparable, while variation exists among individual subjects25). Thus, the results of experiments dealing with blood BDNF concentrations in humans as a group can be extrapolated to the brain response.
Among neurodevelopmental disorders, autism spectrum disorders (ASD) have been intensively studied in relation to blood BDNF. While elevated blood BDNF has been found in patients with ASD, lower blood BDNF is observed in children developing ASD26). Therefore, it is thought that in ASD, BDNF expression is low during the stages of brain development and becomes high after the brain matures. Interestingly, this phenomenon is also observed in neonatal animals subjected to the stress of maternal separation. In these animals, a decrease in BDNF expression during the developmental period is observed as described above, while BDNF expression increases relative to the normal group after termination of the stress exposure4). Considering the above-mentioned findings, it might be possible that a transient decrease in BDNF expression during development inhibits the maturation of inhibitory neurons, resulting in increased activity of excitatory neurons, leading to greater release of BDNF. However further studies are required to prove this hypothesis.
A consensus view on the relationship between attention deficit hyperactivity disorder (ADHD) and blood BDNF has not yet been established, although there is evidence that indicates a possible relationship between ADHD and declines in BDNF from the developmental period to adulthood27). It has been suggested that the decline in BDNF function affects the development of substantia nigra dopaminergic neurons. For example, a number of studies have shown that BDNF has a strong influence on the survival, development, and differentiation of dopaminergic neurons in the substantia nigra. This evidence complements the hypothesis that the decline in BDNF might be involved in the development of ADHD.
Conclusion
Many studies have revealed the function of BDNF during development. However, the full extent of its role in the development of neural circuits remains unclear. This is primarily because the principles of development of neural circuits themselves are still being elucidated. However, there seems to be no doubt about the important role of BDNF in this process. It is hoped that the ongoing elucidation of the role of BDNF in the developing brain will clarify its relationship to developmental disorders and lead to the discovery of effective methods of treatment and intervention.
References
- Hofer, M. et al. : EMBO J., 9, 2459 (1990).
- Timmusk, T. et al. : Neuron, 10, 475 (1993).
- Pöyhönen, S. et al. : Front. Physiol., 10, 486 (2019).
- Ohta, K. I. et al. : J. Neurochem., 141, 179 (2017).
- Huang, Z. J. et al. : Cell, 98, 739 (1999).
- Zheleznyakova, G. Y. et al. : Behav. Brain Funct., 12, 17 (2016).
- Zhang, Y, et al. : Biomed. Pharmacother., 80, 207 (2016).
- Lee, S. T. et al. : Ann. Neurol., 72, 269 (2012).
- West, A. E. et al. : Handb. Exp. Pharmacol., 220, 67 (2014).
- Deinhardt, K. and Chao, M. V. : Neuropharmacology, 76, 603 (2014).
- Numakawa, T. et al. : Histol. Histopathol., 25, 237 (2010).
- Zagrebelsky, M. and Korte, M. : Neuropharmacology, 76, 628 (2014).
- Choo, M. et al. : Nat. Commun., 8, 195 (2017).
- Jones, K. R. et al. : Cell, 76, 989 (1994).
- Cunha, C. et al. : Front. Mol. Neurosci., 3, 1 (2010).
- Cohen-Cory, S. et al. : Dev. Neurobiol., 70, 271 (2010).
- Harward, S. C. et al. : Nature, 538, 99 (2016).
- Horch, H. W. et al. : Neuron, 23, 353 (1999).
- Horch, H. W. and Katz, L. C. : Nat. Neurosci., 5, 1177 (2002).
- Itami, C. et al. : J. Neurosci., 27, 2241 (2007).
- Kimura, F. and Itami, C. : J. Neurosci., 39, 3784 (2019).
- Xenos, D. et al. : Cereb. Cortex, 28, 3399 (2018).
- Lu, Y. et al. : Neurobiol. Learn. Mem., 89, 312 (2008).
- Miao, Z. et al . : Int. J. Mol. Sci ., 21, 1375 (2020).
- Duff y, H. B. D. and Roth, T. : Front. Hum. Neurosci., 14, 594244 (2020).
- Skogstrand, K. et al. : Transl. Psychiatry, 9, 252 (2019).
- Galvez-Contreras, A. Y. et al . : Front. Psychiatry, 8, 126 (2017).




