A Brief Review of Adiponectin and Shibayagi's Mouse/Rat High Molecular Weight Adiponectin ELISA KIT
This article was written by Katsumi WAKABAYASHI, Ph.D. Prof. Emer. Gunma Univ. Technical Consultant, Shibayagi, Co. Ltd..
Adiponectin is an important cytokine secreted from adipocyte i.e. adipokines. It controls lipid metabolism and insulin sensitivity, showing anti-diabetic, anti-atherogenic, and anti-inflammatory actions. It is activated by insulin (1-3).
Chenges of circulating adiponectin observed in obesity, type 2 diabetes and cardiac diseases.
In human subjects, the plasma levels of adiponectin of DM2 patients are lower than those of normal subjects. The levels are further lowered in patients DM2 with coronary artery diseases, namely, DM2(CAD+)
Adiponectin plasma levels are lowered by obesity, and uplifted by body weight decrease, indicating feedback inhibition by obesity (4-6). In women, the blood adiponectin levels are higher than those in men (4). In rhesus monkeys circulating adiponectin levels are lowered in obesity and DM2, and an inverse correlation is observed between plasma adiponectin levels and insulin resistance shown by the activity of insulin on peripheral glucose uptake (7).
The genes related to plasma adiponectin levels are also related to metabolic syndrome, DM2 and risk factors of cardiac diseases (8).
Another important point, i.e. relationship between adiponectin action and its molecular structure will be described later.
Expression of adiponectin gene
Expression of adiponectin is suppressed by glucocorticoid, and enhanced by insulin,IGF-1, and PPARgagonists (9). It is also strongly suppressed byb-adrenergic agents such as catecholamines via G(S)-protein-PKA-dependent route, and may cause catecholamine- induced insulin resistance (10).
Precursor structure (PubMed Q60994)
Mouse adiponectin is composed of 247 amino acids, and the N-terminal 17 amino acids form the signal peptide. The single chain from 18 to 247 forms mature adiponectin, including one complement C1q domain and one collagen-like domain (11-14).
| 1 | mlllqallfl | lilpshaedd | vttteelapa | lvpppkgtca | gwmagipghp | ghngtpgrdg | |
| 61 | rdgtpgekge | kgdagllgpk | getgdvgmtg | aegprgfpgt | pgrkgepgea | aymyrsafsv | |
| 121 | gletrvtvpn | vpirftkify | nqqnhydgst | gkfycnipgl | yyfsyhitvy | mkdvkvslfk | |
| 181 | kdkavlftyd | qyqeknvdqa | sgsvllhlev | gdqvwlqvyg | dgdhnglyad | nvndstftgf | |
| 241 | llyhdtn |
45-110: Collagen-like domain
48-107: Collagen region having repeating sequence of one glycine in 3 amino acids specific to triple helix structure serving as the rod part of C1q component of complement.
Prolines at 47,50,56, and 94 become 4-hydroxyproline after translation, and are important for stabilizing triple helix formation.
112-247: The domain corresponding to globular part of C1q, and contains 8 b-strands(underlined area).
Lysine at 68, 71, 80, and 104 are transformed to 5-hydroxylysine after translation. To these hydroxyl groups, disaccharide, Glc-Gal will be connected as O-linked glycoside. No N-glycoside was found in the molecule.
Complex structure
The monomer form of adiponectin is found only in adipocytes, and homomultimer, i.e. trimer, hexamer and 12-18-mers formed from adiponectin monomer are found in plasma.
Trimer (low molecular weight complexes/LMW)is formed by interaction of non-covalent bonds of triple helix area and by hydrophobic interaction between globular Cq1 domain, and hexamer (middle molecular weight complexes/MMW) or larger complexes (higher molecular weight/HMW)are formed through disulfide bonds between cysteines at 39 of LMW-complexes.
The formation of HMW-complex is also depending upon hydroxylation of lysine and glycosylation (15-17). HMW-complex is more glycosylated than other smaller complexes, and hydrozylation of lysine located in the collagen-like domain and its glycosylation play important roles in the formation and control of secretion of HMW-complex.
It has been reported that the plasma HMW-complex levels reflect the situation of BMI, sex, body weight decrease, glucose tolerance, and insulin sensitivity of the liver better than total adiponectin levels (18,19).
There observed sexual difference in plasma HMW levels, i.e. higher in females than males. HMW levels are increased by orchidectomy. Testosterone administration to castrated males suppresses HMW secretion and decreases the plasma level (20).
Mechanism of action
Expression of adiponectin mRNA is enhanced by administration of thiazolidine derivatives, such as pioglytazone hydrochloride, which are the ligand of PPARg (peroxisome proliferator-activated receptor gamma), and as a result, plasma adiponectin level is increased.
TNF-a, on the other hand, is produced in adipocytes in a high insulin resistant state, and suppresses the expression of adiponectin. PPARg ligand restores this suppression by TNF-a (21-23). Adiponectin is a possible biomarker of in vivo PPARg activity. In an experiment using adiponectin defficient mice, it is suggested that adiponectin is an important factor relating to the recovery of glucose tolerance by PPARg through AMPK (AMP-activated protein kinase) pathway (24).
Adiponectin stimulates and activates phosphorylation of AMPK, enhancing glucose utilization and fatty acid combustion (25,26).
Adiponectin is antagonistic to TNF-a, namely, suppresses ANF-a in liver and macrophage, and also counteracts TNF-a action.
Adiponectin suppresses NF-kB signaling of endotherial cells through cAMP-dependent pathway (27).
Adiponectin suppresses atheroscrelosis by inhibiting expression of adhessive substance and production of macrophage cytokines (21).
Adiponectin is believed to influence on cell growth, angiogenesis, and tissue remodeling of tussues by isolating various growth factors through binding with distinct affinity. The affinity is dependent upon the shape of complexes, LMW, MMW and HMW.
LMW, MMW, and HMW bind HBEGF (#1).
MMW and HMW bind PDGFB (#2), and HMW binds FGF2 (#3) (28).
#1 HBEGF (heparin-binding epidermal growth factor)
Through receptor binding, promotes proliferation of various epidermal cells and fibroblasts. It is necessary in the formation and keeping of the heart. In squamous cell carcinoma EGF receptors are markedly increased. In human ovarian carcinoma, HBEGF is highly expressed.
#2 PDGF (platelet-derived growth factor beta polypeptide)
PDGF is present in a granules of platelets, and released at activation of platelets. It acts on the recovery process of wound by causing cell proliferation in the area of wounded tissue. It is also known as one of the promoting factors of arteriosclerosis.
#3 FGF2(fibroblast growth factor-2, basic fibroblast growth factor, HBGF-2, bFGF)
FGF2 is found in hypophysis, kidney, smooth muscle, cancer tissue, etc. It induces mesoderm during development,, and enhances differentiation of nervous cells, neovascularization, proliferation of blood vessel endotherial cells, and controls proliferation of kidney mesangial cells. Though FGF2 is not found in normal human plasma, FGF appears in the plasma of pituitary tumor patients. Urinary excretion is also increased in cancer patients.
Activation of AMPK in the hypothalamus enhances appetite. Leptin, which is thought to suppress appetite decreases the activity of AMPK in the hypothalamus, and ghrelin, on the other hand, activates hypothalamic AMPK. It has been reported that a pharmacological AMPK activating agent, 5-amino-4-imidazole carboxyamide riboside, when injected into the third ventricle or paraventricular nucleus, significantly stimulated food intake (29)
Two types of adiponectinreceptors have been found, AdipoR1 and AdipoR2.
AdipoR1 is expressed in many tissues including skeletal muscles, while Adipo2 is mostly expressed in liver. Both receptors are found in blood vessels, macrophages, and brain. Adiponectin activates AMPK via AdipoR1 in the arcuate hypothalamus, causing enhancement of food intake and decrease in energy expenditure. In fasting animals, adiponectin levels in plasma and cerebrospinal fluid is increased, and the expression of AdipoR1 in the hypothalamus is also enhanced, and these are lowered after refeeding. These facts indicate that adiponectin is working for saving energy expenditure in the central nervous system in a fasting state (30).
Introduction to Shibayagi's Mouse/Rat High Molecular Weight Adiponectin ELISA KIT
Specificity of the kit
Shibayagi's assay kit for mouse/rat high molecular weight adiponectin is specific to high molecular weight (HMW) complex. Adiponectin HMW complex reflects metabolic syndrome and DM2 better than other complexes.So, measurement of HMW would be more helpful in the analysis of the syndrome than measurement of total adiponectin.
Shibayagi's Mouse/Rat High Molecular Weight Adiponectin ELISA KIT is highly specific to adiponecti HMW as shown in the table and the figure below.
| Species | Substances | Reactivity(%) |
|---|---|---|
| Mouse | Adiponectin(HMW) | 100 |
| Adiponectin(Hexamer) | < 5 | |
| Adiponectin(Trimer) | - | |
| Adiponectin(Monomer) | - | |
| MCH | - | |
| TNF-a | - | |
| INFg | - | |
| Insulin | - | |
| Leptin | - | |
| Rat | Adiponectin(HMW) | 100 |
| Adiponectin(Monomer) | - | |
| TNF-a | - | |
| INFg | - | |
| Insulin | - | |
| Leptin | - |
Molecular size specificity of the Mouse/Rat High Molecular Weight Adiponectin ELISA KIT by mouse serum fractionation
Condition of Fractionation
System: AKTA explorer 100s (Pharmacia Biotech)
Column: HiPrep 26/60 Sephacryl S-300 (Pharmacia Biotech)
Elution: 50mM PBS pH 7.2
Wavelength: 280 nm
Flow rate: 2ml/min
Sample: Mouse serum: Balb/c, 6w, male
Loaded volume of the sample: 1.5ml
Absorbance of every fraction was measured at 280nm.
Then each fraction was assayed with Shibayagi's HMW kit (absorbance 450-620nm) and also with commercially available kit for "total adiponectin" (absorbance at 450nm).
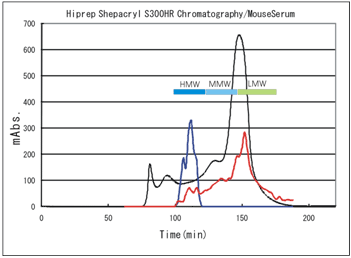
--- : Abs. at 280nm showing the amount of total protein Absorbance when each fraction was assayed by,
--- : Shibayagi's kit(450-620nm), --- : Commercial total kit(450nm)
It is clearly shown that Shibayagi's kit measures only HMW.
Brief description about the kit and operation
Advantage
- Rapid assay (total reaction time: 4 hours).
- A small sample volume.
- An ecologically excellent preservative is used.
- Every reagent is provided in liquid form and ready to use.
- Excellent precision and reproducibility.
Components
| Reagents | Amounts | |
| (A) | Anti-adiponectin-coated plate | 96 wells(8x12) / 1 plate |
| (B) | Standard adiponectin solution (2000ng/ml) | 200ml / 1 vial |
| (C) | Buffer solution | 60ml/1 bottle |
| (D) | HRP-conjugated anti-adiponectin | 100ml/ 1 vial |
| (F) | Chromogenic substrate reagent (TMB) | 12ml/ 1 bottle |
| (H) | Reaction stopper (1M H2SO4) | 12ml/ 1 bottle |
| (I) | Concentrated washing buffer (10x) | 100ml/ 1 bottle |
Assay sample
Rat or mouse serum or plasma : 10ml in the standard procedure.
In most cases, samples should be diluted to 25-50X using assay buffer (C) as shown in the table below.
| 25-fold dilution | 50-fold dilution | |
| Serum/Plasma (ml) | 10 | 10 |
| Buffer C (ml) | 240 | 490 |
Assay range
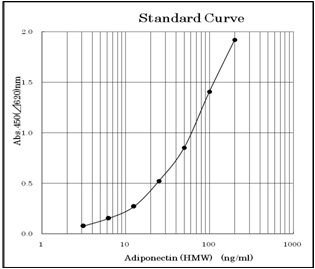
3.13 ~ 200ng/ml (Standard curve range)
(In the case of 50X dilution, original plasma HMW adiponectin range is 156~10,000 ng/ml)
Summary of Assay Procedure

An example of standard curve
Storage condition
Store the kit at 2~8C. Do not freeze.
Term of validity
Six months from production. Expiration date is indicated on the container.
Unit of package
96 wells/1 plate
Product code
AKMAN-11
Kit validation and assay data for rat/mouse high molecular weight adiponectin
Assay validation data
Assay precision (intra-assay variation)
| Well | Sample A | Sample B |
| 1 | 29.5 | 129 |
| 2 | 30.7 | 125 |
| 3 | 29.8 | 128 |
| 4 | 29.0 | 126 |
| 5 | 29.6 | 126 |
| mean. | 29.7 | 127 |
| SD | 0.631 | 1.89 |
| CV(%) | 2.12 | 1.49 |
Unit:ng/ml
Reproducibility (inter-assay variation)
| Samples | Day 1 | Day 2 | Day 3 | Day 4 | mean. | SD | CV(%) |
| C | 196 | 192 | 196 | 190 | 193 | 2.63 | 1.36 |
| D | 126 | 130 | 125 | 125 | 127 | 2.27 | 1.79 |
| E | 62.5 | 59.1 | 60.7 | 60.3 | 60.7 | 1.41 | 2.33 |
Unit: ng/ml n=2
Recovery test
| Sample No.H | Sample No.I | ||||||
| Added | Found | Recovered | Recovery (%) | Added | Found | Recovered | Recovery (%) |
| 0.00 | 68.5 | - | - | 0.00 | 23.3 | - | - |
| 35.0 | 103 | 34.5 | 98.6 | 18.0 | 40.3 | 17.0 | 94.4 |
| 65.0 | 132 | 63.5 | 97.7 | 26.0 | 50.6 | 27.3 | 105 |
| 95.0 | 165 | 96.9 | 102 | 32.0 | 55.5 | 32.2 | 101 |
Unit: ng/ml n=2
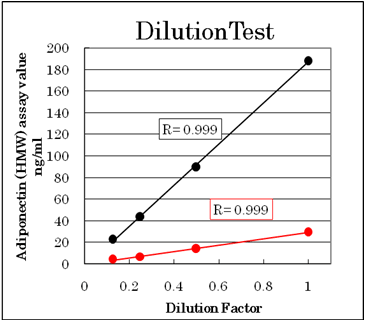
Dilution test
Mouse assay data
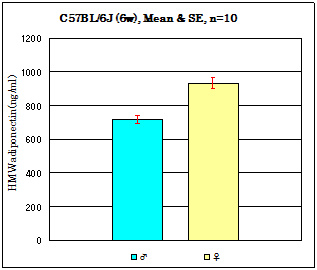
Strain: C57BL/6J Age: 6 weeks Fasting: 24hours
Sample: Serum/ Isoflurane anesthesia/Heart puncture
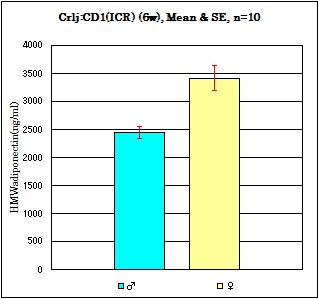
Strain: Crli:CD1(ICR) Age: 6 weeks Fasting: 24hours
Sample: Serum/Iisoflurane anesthesia/Heart puncture
Effect of pioglitazone administration on serum HMW adiponectin levels
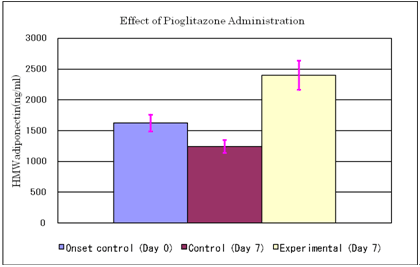
Results are expressed as Mean & SE
Animals: KK-Ay/Ta mouse
Twelve mice were fed on high fat diet (20% soybean oil added to CE-2 chow) from day -4, and were divided into experimental and control groups each consisted of 6 animals. Pioglitazone administration to the experimental group: 10mg/10ml/kg p.o. (n=6)
Physiological saline to the control group (n=6)
Blood sampling on day 0 (onset control, n=12) and day 7
Mean body weight of the experimental group: 38.3g
Mean body weight of the control group: 38.0g
Assay data for other mouse strains
| Strain | Age | n | Sex | Mean, (ng/ml) |
SD, (ng/ml) |
|---|---|---|---|---|---|
| Balb/c | 6w | 10 | male | 2369 | 743 |
| ICR | 6w | 10 | male | 2119 | 802 |
| BKS.Cg-(+Lepdb9/Lepdb) | 12w | 10 | male | 1802 | 875 |
| B6.V-Lepob/J | 12w | 10 | male | 1229 | 437 |
Serum samples, free access to food and water.
Rat assay data
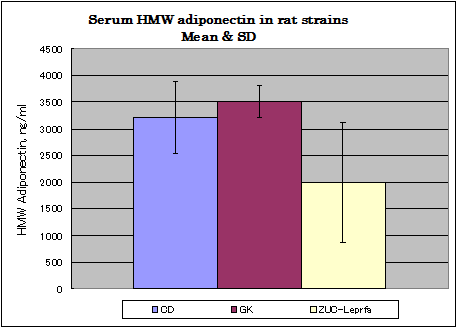
Normal diet,Afree access to food and water.
CD: ( 8w, males, n=8), GK :(12w, males, n=12) Zuc-Leprfa: (8w, males, n=8)
K.W. 09/01/13
References
- Yamauchi,T. et al Nat. Med. 7 (8), 941-946 (2001)
- Berg,A.H., Combs,T.P., Du,X., Brownlee,M. and Scherer,P.E. Nat. Med. 7 (8), 947-953 (2001)
- Xu,A., Wang,Y., Keshaw,H., Xu,L.Y., Lam,K.S. and Cooper,G.J. J. Clin. Invest. 112 (1), 91-100 (2003)
- Hotta, K., et al. Arterioscler Thromb Vas Biol 20(6), 1595-1599 (2000)
- Weyer, C., et al. J Clin Endocrinol Metab 86(5), 1930-1935 (2001)
- Wei-Shiung Y., et al. J Clin Endocrinol Metab 86(8), 3815-3819 (2001)
- Hotta, K. et al. Diabetes 50(5), 1126-1133 (2001)
- Comussie, A.G., et al. Human Biology 79(2), 191-200 (2007)
- Halleux, C.M., et al. Biochem Biophys Res Commun 288(5), 1102-1107 (2001)
- Fasshauser, M., et al. FEBS Lett 507(2), 142-146 (2001)
- Scherer,P.E., Williams,S., Fogliano,M., Baldini,G. and Lodish,H.F. J. Biol. Chem. 270 (45), 26746-26749 (1995)
- Hu,E., Liang,P. and Spiegelman,B.M. J. Biol. Chem. 271 (18), 10697-10703 (1996)
- Das,K., et al. Biochem. Biophys. Res. Commun. 280 (4), 1120-1129 (2001)
- Carninci,P., et al. Science 309 (5740), 1559-1563 (2005)
- Wang,Y., Xu,A., Knight,C., Xu,L.Y. and Cooper,G.J. J. Biol. Chem. 277 (22), 19521-19529 (2002)
- Wang,Y. et al J. Biol. Chem. 281 (24), 16391-16400 (2006)
- Richards, A. A. , et al. Mol Endocrinol. 20(7), 1673-1687 (2006)
- Sinha, M.K., et al. Clinical Chemistry 53, 2144-2151 (2007)
- Fisher, F.F. et al. Diabetologia 48(6), 1084-1087
- Xu,A., et al. J. Biol. Chem. 280 (18), 18073-18080 (2005)
- Maeda, N., et al. Diabetes 50(9), 2094-2099
- Pajvani, U.B. et al. J Biol Chem 2003 Mar 14;278(11):9073-85 (2003).
- Combs T.P., et al. Endocrinology 143 (3):998-1007 (2002)
- Nawrocki, A.R., et al. J Biol Chem 281(5), 2654-60 (2006)
- Ofir,M. et al. Harefuah 146(10), 770-775 (2007)
- Yoon, M.J., et al. Diabetes 55 (9), 2562-2570 (2006)
- Ouchi, N., et al. Circulation 102(11), 1296-301 (2000)
- Wang,Y., et al. J. Biol. Chem. 280 (18), 18341-18347 (2005)
- Andersson, U., et al. J Biol Chem 279(13):12005-12008 (2004)
- Kubota, N. et al. D Cell Metab 6 (1),55-68 (2007)
Adiponectins of various animals
Human(PubMed NP_004744)
| 1 | mlllgavlll | lalpghdqet | ttqgpgvllp | lpkgactgwm | agipghpghn | gapgrdgrdg | |
| 61 | tpgekgekgd | pgligpkgdi | getgvpgaeg | prgfpgiqgr | kgepgegayv | yrsafsvgle | |
| 121 | tyvtipnmpi | rftkifynqq | nhydgstgkf | hcnipglyyf | ayhitvymkd | vkvslfkkdk | |
| 181 | amlftydqyq | ennvdqasgs | vllhlevgdq | vwlqvygege | rnglyadndn | dstftgflly | |
| 241 | hdtn |
Rhesus monkey(NP_001028043)
| 1 | mllgavllll | alpshgqdtt | tqgpgvllpl | pkgactgwma | gipghpghng | vpgrdgrdgt | |
| 61 | pgekgekgdp | gligpkgdtg | etgvtgaegp | rgfpgiqgrk | gepgegayvy | rsafsvglet | |
| 121 | yvtvpnmpir | ftkifynqqn | hydgstgkfh | cnipglyyfa | yhitvymkdv | kvslfkkdka | |
| 181 | mlftydqyqe | nnvdqasgsv | llhlevgdqv | wlqvygeger | nglyadndnd | stftgfllyh | |
| 241 | dtn |
Cattle (NP_777167)
| 1 | mllqgallll | lalpshgedn | medpplpkga | cagwmagipg | hpghngtpgr | dgrdgtpgek | |
| 61 | gekgdagllg | pkgetgdvgm | tgaegprgfp | gtpgrkgepg | eaayvyrsaf | svgletrvtv | |
| 121 | pnvpirftki | fynqqnhydg | stgkfycnip | glyyfsyhit | vymkdvkvsl | fkkdkavlft | |
| 181 | ydqyqeknvd | qasgsvllhl | evgdqvwlqv | yegenhngvy | adnvndstft | gfllyhnive |
Pig (NP_999535)
| 1 | mlllgavlll | lalpslgqet | tekpgallpm | pkgacagwma | gipghpghng | tpgrdgrdgv | |
| 61 | pgekgekgxt | gltxpkgdtg | esgvtgvegp | rgfpgipgrk | gepgesayvy | rsafsvglet | |
| 121 | rvtvpnmpir | xtkifynqqn | hydvttgkfh | cnipglyyfs | fhvtvylkdv | kvslykkdka | |
| 181 | vlftydqyqd | knvdqasgsv | llylekgdqv | wlqaygdeen | ngvyadnvnd | siftgfllyh | |
| 241 | nie |
Rabbit (NP_001075691)
| 1 | mlllqavlll | lalpshgqds | ttespgvlip | apkgacagwi | agipghpghn | gtpgrdgrdg | |
| 61 | tpgekgekgd | aglvgpkgdt | getgvtgaeg | prgfpgspgr | kgepgegayv | yrsafsvgle | |
| 121 | grvtipnvpi | rftkifynqq | nhydsttgkf | rcnipglyyf | syhitvymkd | vkvslfkkdk | |
| 181 | amlftydqyq | dknvdqasgs | vllhlqvddq | vwlqvygdgd | hnglyadnvn | dsistgflly | |
| 241 | hdtn |
Cat (BAF52934)
| 1 | mlllravlll | lvlpirgqds | etegpgvvvp | lpkgactgwm | agipghpghn | gtpgrdgrdg | |
| 61 | tpgekgekgd | pglvgpkgdt | getgvtgieg | prgfpgipgr | kgepgesayv | yrsafsvgle | |
| 121 | srvtvpnvpi | rftkifynqq | nhydvttrkf | hcnipglyyf | syhitvylkd | vkvslykrdk | |
| 181 | amlftydqyq | eknvdqasgs | vllhletgde | vwlqvygdgd | ynglyadnvn | dstftgflly | |
| 241 | ydtv |
Chicken (AAX40986)
| 1 | mrgsvgfllc | slllalsgte | madqadqsdp | kmscanwmgg | apghpghngl | pgrdgkdgkd | |
| 61 | gqkgdkgepg | lqgvkggtge | kgatgaegpr | gfpghmgmkg | qkgessyvyr | safsvglter | |
| 121 | aphpnvpirf | tkifyneqnh | ydsstgkflc | sipgtyffay | hltvymtdvk | vslykkdkav | |
| 181 | iftydqfqen | nvdqasgsvl | lhlslgdevw | lqvygegnnn | gvyadninds | tfmgfllypd | |
| 241 | tddr |




