1st review: Neuropathic pain
This article was written by Dr. Makoto Tsuda, Department of Life Innovation, Graduate School of Pharmaceutical Sciences, Kyushu University.
Introduction
In response to harmful stimuli, pain occurs in the body through the nociceptive system to trigger defensive behavior. However, chronic pain called neuropathic pain occurs when the nervous system is damaged, compressed, or dysfunctional due to cancer, diabetes mellitus, cerebral infarction, spinal cord injury, healed herpes zoster, etc. Spontaneous pain, hyperalgesia (enhanced pain by a stimulus that normally provokes pain), and allodynia (pain resulting from normally non-noxious stimuli such as tactile stimuli) are known as typical symptoms of neuropathic pain. These symptoms may be due to dysfunction of the somatosensory communication system rather than simply due to continuous pain signals for biological defense. Mechanisms underlying abnormalities in the neurotransmission systems starting in peripheral tissues, passing through primary afferent neurons and then spinal dorsal horns, and finally reaching the brain have not been elucidated. This article briefly describes the important role of microglia in abnormal pain transmission in the spinal cord and brain, and introduces a potential mechanism of neuropathic pain and offers future perspectives.
Microglia
Microglia are one type of glial cells, which are a component of the central nervous system. Adult microglia are found in the form of a small cell body with multiple fine branching processes (Fig. 1). In vivo imaging of brain microglia revealed that microglia are engaged in spatiotemporally very dynamic activities, including responses to synaptic activity and cytotoxicity, by continuously moving their processes.1)
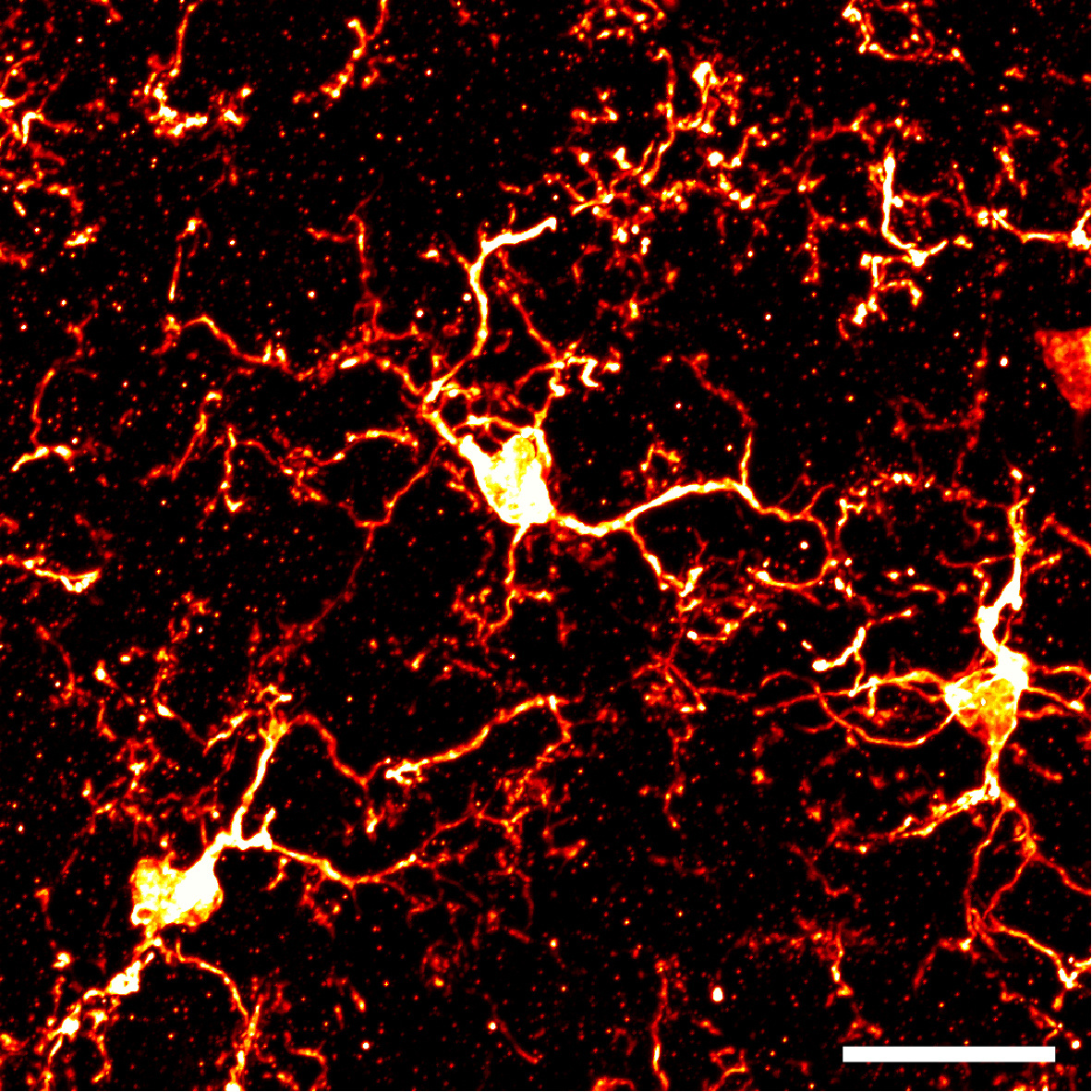
| Figure 1. Microglia in normal state (spinal dorsal horn) Immunohistological staining with anti-IBA1 antibody. Scale bar: 50 μm |
Fate-mapping studies in 2010 and later have shown that microglia originate from embryonic yolk sac progenitors, which migrate to the brain through blood circulation to differentiate into mature microglia,2) and microglia are currently classified as tissue-resident macrophages in the central nervous system. It is hypothesized that adult microglia are maintained by self-renewal, rather than arise from the bone marrow as previously assumed. Since deficiency of colony stimulating factor 1 receptor (CSF1R),3) treatment with CSF1R inhibitors,4) and deficiency of interleukin-34 (IL-34)5) result in reduction or loss of microglia, CSF1R and IL-34 may be important signals, although the details are still unclear.
Microglia in the spinal dorsal horn
Animal models with directly injured peripheral nerves (especially sciatic or spinal nerves) are used in basic research of neuropathic pain. It was reported in the 1970s that sciatic axotomy activated microglia in the dorsal horn of spinal cord, the temporal correlation between neuropathic pain and microglial activation was shown in the 1990s, and the causality between the two was first revealed in 2003.6) More recently, microglia have attracted attention and been extensively studied as a potential mechanism of neuropathic pain in the world. Molecular and cellular mechanisms identified to date are briefly described below.
Mechanism of microglial activation
Peripheral nerve injury triggers dramatic cellular responses of dorsal horn microglia, including cell body hypertrophy, process retraction and thickening, and rapid increase in cell number.7) The nerve injury-induced increase in cell number results from self-proliferation of microglia through cell division. A molecular mechanism underlying this process might be that CSF1 overexpressed in injured neurons in a dual leucine zipper kinase (DLK)-dependent manner is axonally transported to the spinal dorsal horn and acts on CSF1R expressed in microglia8, 9) (Fig. 2). It is known that infiltration of peripheral blood monocytes/macrophages is observed in the brain and/or spinal cord of neurological disease models in which the blood-brain barrier function is impaired. In neuropathic pain models, nerve injury results in a transient reduction in blood-spinal cord barrier function, but not infiltration of peripheral blood monocytes/macrophages in the spinal dorsal horn.10)
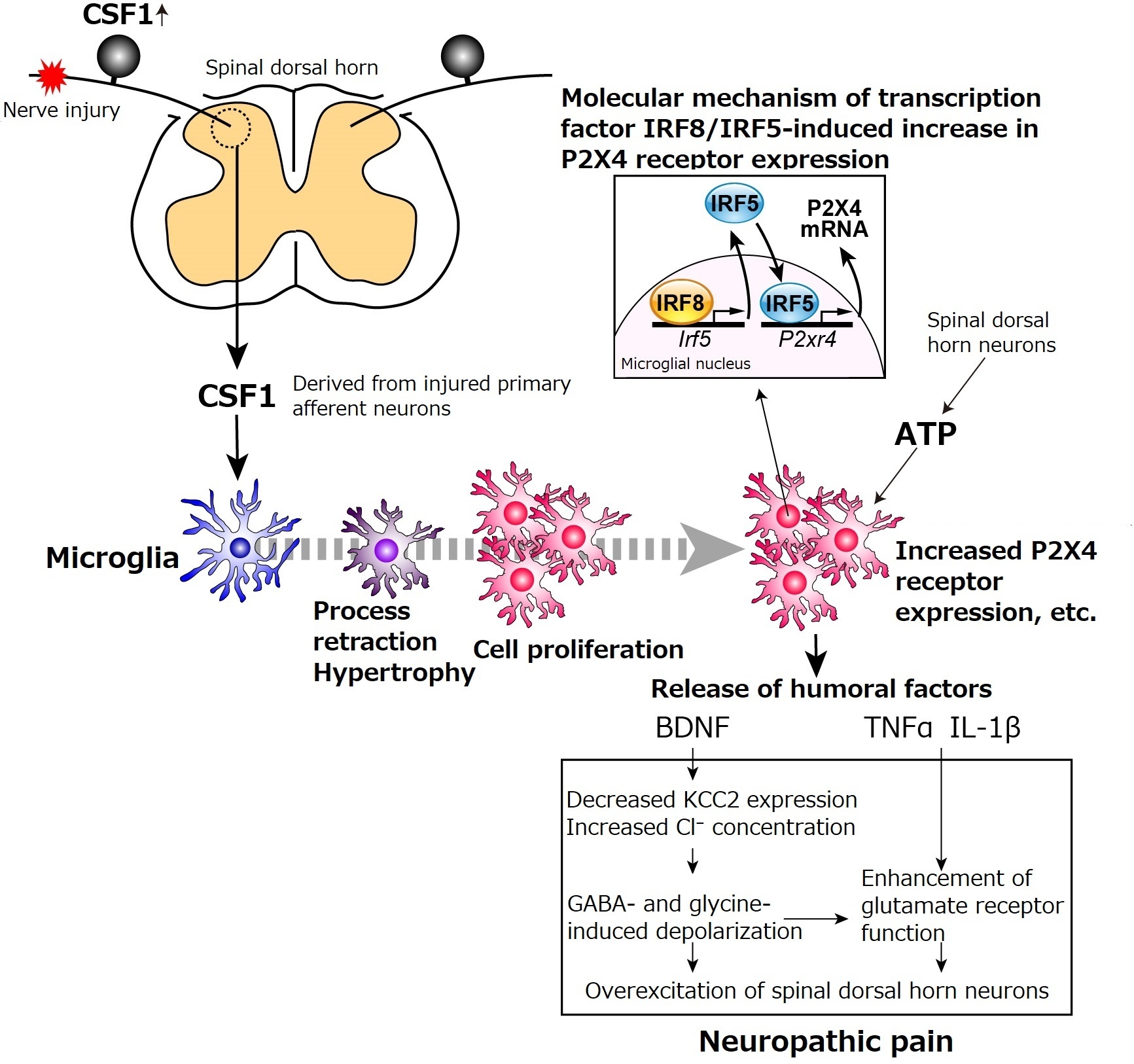
| Figure 2. Mechanisms of microglial activation in the spinal dorsal horn and change in nerve function CSF1 overexpressed in injured primary afferent neurons acts on CSF1 receptor in dorsal horn microglia to activate microglia along with morphological changes, cell proliferation, and gene expression. P2X4 receptor expression is increased through the transcription factor IRF8-IRF5 cascade. P2X4 receptor is stimulated by ATP released from spinal dorsal horn neurons to stimulate microglial release of humoral factors. BDNFs increase intracellular Cl- concentrations by decreasing KCC2 expression to induce the excitatory actions of GABA and glycine. In addition to this neuronal excitation, TNFα and IL-1β enhance glutamate receptor function to cause overexcitation of spinal dorsal horn neurons, leading to neuropathic pain. |
Mechanism of activated spinal microglia-induced changes in somatosensory information processing
Activated microglia undergo epigenetic changes,11) resulting in functional activation through expression of various genes, including cell membrane receptors, intracellular protein kinases, and humoral factors.6) One of the most representative molecules is receptors for extracellular nucleotides such as ATP (P2 receptors). Nerve injury results in a microglia-specific increase in the expression of ionotropic P2X4 and P2X7 receptors and G-protein-coupled P2Y12 receptor in the spinal cord, and inhibition of function and expression of these receptors results in marked inhibition of allodynia.6, 12) P2X4 receptor expression is increased by transcription factors IRF8 and IRF5.13, 14) This receptor is activated by ATP released from spinal dorsal horn neurons15) to stimulate the production and release of humoral factors such as brain-derived neurotrophic factor (BDNF). BDNF reduces the expression of K+-Cl- cotransporter 2 (KCC2) in spinal dorsal horn neurons, which are involved in pain transmission to the brain, to collapse the intra- to extracellular concentration gradient of Cl- and thereby induce the excitatory actions of γ-aminobutyric acid (GABA) and glycine16). The neuronal excitation also leads to activation of N-methyl-D-aspartate (NMDA) receptors. The abnormal excitation of spinal dorsal horn neurons is responsible for the nerve injury-induced neuropathic allodynia (Fig. 2).6)
Microglia are principal inflammatory cytokine-producing cells in the brain and spinal cord. Triggering receptor expressed on myeloid cells 2 (TREM2)/DNAX-activating protein of 12 kDa (DAP12), toll-like receptors (TLRs), and Nod-like receptor NACHT-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasomes mediate the production of IL-1β, which enhances glutamate receptor function and inhibits GABA and glycine receptor function in spinal dorsal horn neurons.6) Tumor necrosis factor (TNF) α is selectively expressed in microglia and enhances the neuronal excitability through its direct effects on spinal dorsal horn neurons and indirect effects on astrocytes and vascular endothelial cells. TNFα also acts on microglia to increase BDNF expression and thereby increase the spine formation and synaptic connection of pain transmission neurons.17) In addition, the platelet-activating factor (PAF) released from microglia stimulates the microglial PAF receptor in an autocrine manner to induce PAF production, forming a positive loop to contribute to neuropathic pain.18)
Brain microglia
It is reported that peripheral nerve injury also results in microglial activation in several brain regions, which is not morphologically as dramatic as activation of dorsal horn microglia. Microglia impair the function of mesolimbic dopaminergic neurons and inhibit the reward system when activated in the ventral tegmental area controlling the reward system,19) and reduce the spine density, synaptic transmission, and BDNF expression in CA1 pyramidal neurons and impair memory when activated in the hippocampus.17) In the central nucleus of the amygdala, on the other hand, peripheral blood monocytes/macrophages infiltrate relatively as late as 4 weeks after nerve injury to contribute to NMDA receptor phosphorylation and anxiety behavior.20) Taken together, microglia activated in the brain may be involved in emotional and memory aspects of neuropathic pain.
Application to treatment and diagnosis
Compounds and antibodies targeting the molecules in microglia, including P2X4 receptor, have been developed and reported to be effective in preclinical studies.6, 21) It is hoped that the efficacy will also be shown in clinical studies in patients with neuropathic pain.
Microglial imaging techniques in the human brain and spinal cord are applicable to drug discovery, treatment, and even diagnosis. While microglia are extremely difficult to biopsy, a research group led by Takahiro Kato at Kyushu University found that induced microglia-like cells (iMG cells) differentiated from peripheral blood monocytes of patients with fibromyalgia had an increased ability to release TNFα, and this increase was associated with pain.22) Future studies may be expected to lead to application of iMG cells to diagnosis and treatment of chronic pain.
Conclusion
Many studies over the past several decades have revealed the importance of not only neurons, but also microglia, which are activated in the spinal dorsal horn and brain along with neuronal damage, in the development of neuropathic pain.6) Contribution of astrocytes to neuropathic pain has also been shown (refer to another review,23) etc.). These findings serve as an important example of powerful effects of glial cells, which have traditionally been regarded merely as nerve supporting cells, on neuronal function, highlighting the importance of perspective to neuron-glia interactions to understand the mechanism of neuropathic pain and central nervous functions. On the other hand, it is very unclear how the heterogeneity of microglia, which has been revealed by recent single-cell analyses, is altered after neuronal damage, and what this alteration means in chronic pain, requiring further studies. Future clarification of roles of microglial subsets and microglial interactions with neurons may open an important way to elucidate the mechanism of neuropathic pain and develop treatments.
References
- Wake, H. et al. : Trends Neurosci., 36, 209 (2013).
- Prinz, M. et al. : Cell, 179, 292 (2019).
- Erblich, B. et al . : PLoS One , 6, e26317 (2011).
- Elmore, M. R. et al. : Neuron, 82, 380 (2014).
- Wang, Y. et al . : Nat. Immunol., 13, 753 (2012).
- Inoue, K. and Tsuda, M. : Nat. Rev. Neurosci., 19, 138 (2018).
- Kohno, K. et al. : Biol. Pharm. Bull., 41, 1096 (2018).
- Guan, Z. et al. : Nat. Neurosci., 19, 94 (2016).
- Wlaschin, J. J. et al. : Elife, 7, (2018).
- Tashima, R. et al. : Sci. Rep., 6, 23701 (2016).
- Denk, F. et al. : Cell Rep., 15, 1771 (2016).
- Tsuda, M. et al. : Nature, 424, 778 (2003).
- Masuda, T. et al. : Cell Rep., 1, 334 (2012).
- Masuda, T. et al. : Nat. Commun., 5, 3771 (2014).
- Masuda, T. et al. : Nat. Commun., 7, 12529 (2016).
- Coull, J. A. et al. : Nature, 438, 1017 (2005).
- Liu, Y. et al. : J. Neurosci., 37, 871 (2017).
- Shindou, H. et al. : Faseb J., 31, 2973 (2017).
- Taylor, A. M. et al. : J. Neurosci., 35, 8442 (2015).
- Sawada, A. et al. : Pain, 155, 1762 (2014).
- Williams, W. A. et al. : Pain, 160, 1989 (2019).
- Ohgidani, M. et al . : Sci. Rep., 7, 11882 (2017).
- Ji, R. R. et al. : Nat. Rev. Neurosci., 20, 667 (2019).




