Arsenic compounds contained in various foods
The original article by Tadashi Kitta of the Japan Food Inspection Corporation, appeared in an e-newsletter of FUJIFILM Wako Pure Chemical Corporation and was translated by the company.
Chemical forms of arsenic and their toxicities
Arsenic (As) is an element occurring in the Earth's crust mainly as sulfides such as As3S3, As4S4, and FeAsS. Because it is released into the environment through natural phenomena such as volcanic activity and mineral weathering, arsenic is widely distributed in the atmosphere, rivers, lakes, oceans, and land. In the natural environment, the major chemical forms of arsenic are the oxygen-coordinated inorganic arsenic forms arsenous acid, or As (III), and arsenic acid, or As (V) (Figure 1). Undergoing metabolic conversion via food chains in the marine ecosystem, these forms of arsenic occur in marine organism tissues mainly as organic arsenic compounds such as monomethylarsonic acid (MMA), dimethylarsinic acid (DMA), arsenobetaine (AsBe), and arsenocholine (AsC) (Figuer 1).
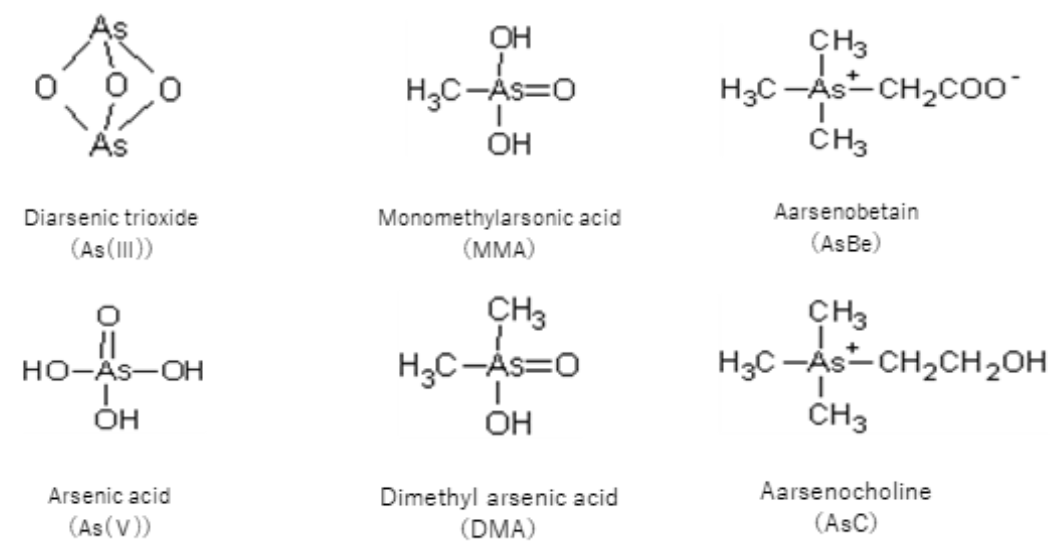
Figure 1 Representative arsenic compounds
The toxicities of arsenic compounds are known to depend largely on their chemical forms. In general, inorganic arsenic exhibit higher toxicity than organic arsenic, and trivalent forms (oxidation number 3) are more toxic than pentavalent forms (oxidation number 5). In acute poisoning by inorganic arsenic, irritation of oral, esophageal, and other mucosal membranes is followed by esophageal pain, after which vomiting, abdominal pain, diarrhea, and other symptoms develop within several minutes to hours. In serious cases, violent vomiting and diarrhea, muscle cramps, cardiomyopathy, renal impairment, and other symptoms develop, and the patient dies within 24 hours at earliest. Although organic arsenic forms reportedly do not exhibit acute toxicity or peripheral nervous system toxicity, MMA (III) and DMA (III) have been suggested to possibly affect living organisms.
In its Monographs on the Identification of Carcinogenic Hazards to Humans, a carcinogenicity assessment conducted in 2004, the International Agency for Research on Cancer (IARC) classified "arsenic in drinking water" under Group 1 (carcinogenic to humans) as there is sufficient evidence for induction of bladder cancer, lung cancer, and skin cancer in humans. In a reevaluation conducted in 2012, inorganic arsenic compounds were classified under Group 1, DMA (V) and MMA (V) under Group 2B (possibly carcinogenic to humans), and AsBe and other organic arsenic compounds that are not metabolized in humans under Group 3 (not classifiable as to their carcinogenicity to humans).
Dietary intake of arsenic
Data on daily per-capita intake of total and inorganic arsenic from a total diet (market basket) study conducted in fiscal 2016-2018 under a MHLW Grant-in-Aid for Scientific Research are shown in Figure 2.
As for the overall intake of total arsenic, seafood (134 μg/man/day, 58.3%), vegetables and seaweeds (72.6 μg/man/day, 31.6%), and rice and processed rice (13.8 μg/man/day, 6.0%) accounted for large proportions. It has been reported that most vegetables have low arsenic content, and total arsenic content in seafood and seaweeds such as kelp and wakame are for the most part organic arsenic compounds of relatively small impact, such as arsenosugars and AsBe.
As for the overall intake of inorganic arsenic, which is highly toxic, rice and its processed products (12.4 μg/man/day, 73.8%) and vegetables and seaweeds (2.2 μg/man/day, 13.1%) accounted for large proportions. The proportion of inorganic arsenic in total arsenic was high at 89.9% in rice and its processed products. This is because rice plants absorb inorganic arsenic while being cultivated in paddy fields for long time periods during which inorganic arsenic in soils is likely to dissolve.
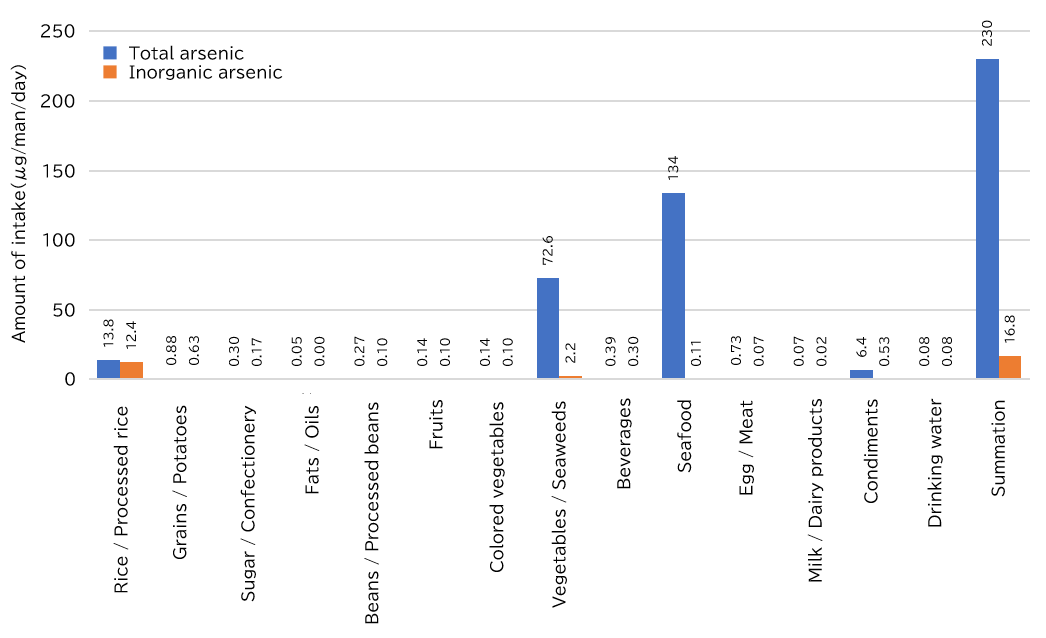
Figure 2 Daily per-capita dietary intake of total arsenic and inorganic arsenic by food group
Chemical form analysis of arsenic in rice
The Codex Alimentarius Commission, an organization working to draw up international food specifications, recognized that rice has higher arsenic concentrations than other agricultural products and thus contributes largely to the oral intake of inorganic arsenic via contaminated irrigation and/or cooking water, and established reference values for maximum limits of inorganic arsenic concentrations in rice (0.2 mg/kg polished rice, 2014; 0.35 mg/kg husked rice, 2016), and then formulated the Code of Practice for the Prevention and Reduction of Arsenic Contamination in Rice (CXC 77-2017) in 2017. Based on this, the Japanese Ministry of Agriculture, Forestry and Fisheries has been conducting actual status surveys on inorganic arsenic content in rice, and is advancing technical development for the reduction of inorganic arsenic.
With this background, it is necessary to separate inorganic and organic arsenic forms found at low concentration levels in rice, and quantify them. For this purpose, HPLC/ICP-MS, a combination of high-performance liquid chromatography (HPLC) and inductively coupled plasma mass spectrometry (ICP-MS), is widely used. The chemical form analysis procedures are designed to avoid changes in the chemical forms of the various arsenic compounds during pretreatment processes such as extraction, heating, and dilution (Figuer 3).

Figure 3 Extraction ion chromatogram of arsenic compounds by HPLC/ICP-MS
HPLC is suitable for the separation of the various arsenic compounds, whereas highly sensitive ICP-MS is suitable for the detection of the separated compounds. The arsenic compounds have different acid dissociation constants and can occur in multiple forms concurrently, such as in anionic, cationic, amphoteric, and non-ionic states, depending on the mobile phase pH conditions; therefore, reversed-phase ion-pairing chromatography is often used, with sodium 1-butanesulfonate as the ion-pairing reagent for the analysis of basic compounds, and tetramethylammonium hydroxide as the ion-pairing reagent for the analysis of acidic compounds (Figure 4). Being highly reliable as validated by inter-laboratory joint experiments, this analysis method is used for contamination status surveys and other purposes.
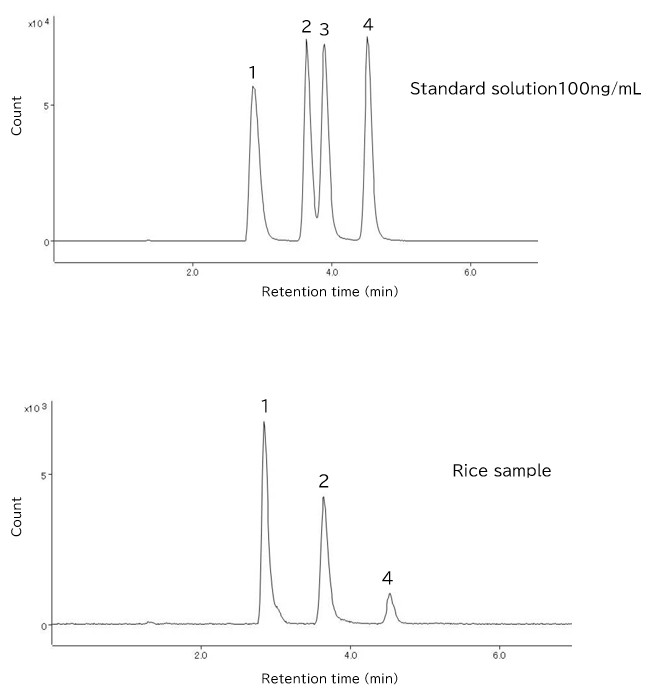
Figure 4 Flow chart for chemical form analysis of arsenic in husked rice
| Column | CAPCELL PAK C18MG (φ4.6 mm * 250 mm, particle size: 5 μm) |
|---|---|
| Mobile phase | 0.05 %(v/v) Methanol, 10 mmol/L Sodium 1-Butanesulfonate, 4 mmol/L Malonic Acid, 4 mmol/L Tetramethylammonium Hydroxide (pH3.0) |
| Flow rate | 0.75 mL/min |
| RF output | 1.55 kW |
| Plasma gas flow rate (Ar) | 15 L/min |
| Carrier gas flow rate (Ar) | 1.0 L/min |
| Auxiliary gas flow rate (Ar) | 0.90 L/min |
| Analyte | 1. As (V), 2. As (III), 3. MMA, 4. DMA |
Conclusion
Although rice and the seaweed "Hijiki" are richer in inorganic arsenic than other foods, it has not been reported that they have adverse effects on health such as causing arsenic poisoning within the normal range of intake based on Japanese food culture. As an assessment conducted by the Food Safety Commission of Japan has concluded, "There is no problem if a balanced dietary lifestyle is maintained."; it is important that we endeavor to have a balanced dietary lifestyle without preference for particular foods.
References
- 化学物質・汚染物質評価書 食品中のヒ素,食品安全委員会(2013).(in Japanease)
- 平成30年度厚生労働行政推進調査事業費補助金 食品の安全確保推進研究事業 食品を介したダイオキシン類等有害物質摂取量の評価とその手法開発に関する研究 総括・分担研究報告書, 厚生労働省(2019). (in Japanese)
- CODE OF PRACTICE FOR THE PREVENTION AND REDUCTION OF ARSENIC CONTAMINATION IN RICE, CXC 77-2017, CODEX(2017).
- コメ中ヒ素の低減対策の確立に向けた手引き, 農林水産省(2019). (in Japanese)
- Preliminary Report On International Validation Of Analytical Method To Determine Inorganic Arsenic In Rice,CCCF 17th Session agenda item 14,CODEX(2013).
- LC/MS,LC/MS/MSの基礎と応用, Ohmsha, Ltd. (2014). (in Japanese)




