Detecting Labware Contamination
This article was written by Dr. Masakazu Tsuchiya, FUJIFILM Wako Pure Chemical Corporation, for Vol. 62 No. 3 (July 1994) of Wako Junyaku Jiho.
The content of this article is from the time of publication. It is not the latest information due to new knowledge and changes in regulatory rules after original publication.
When using plastic labware for Limulus Amebocyte Lysate (LAL) tests, special attention should be given to contamination by endotoxins or other LAL activating substances, as well as, adsorption of endotoxins and samples. This post will discuss a method employed by FUJIFILM Wako to detect endotoxin contamination in labware.
Contaminated plastic labware that cannot be extracted with water or saline can be extracted with protein solutions such as those containing albumin1). This type of contamination poses no threat when measuring samples like water, but can be problematic when working with plasma and other protein samples.
We therefore developed a method for extracting and measuring adsorbed endotoxin by rinsing contaminated labware with a solution of 0.1% Human Serum Albumin (HSA).
An important aspect of developing this method was designing a model of contamination by endotoxin adsorption. It was known from experience that endotoxin can adsorb onto plastic, but this effect was difficult to reproduce quantitatively. Accordingly, it was decided that research should concentrate on how to design a contamination model that could be used to assess extraction performance.
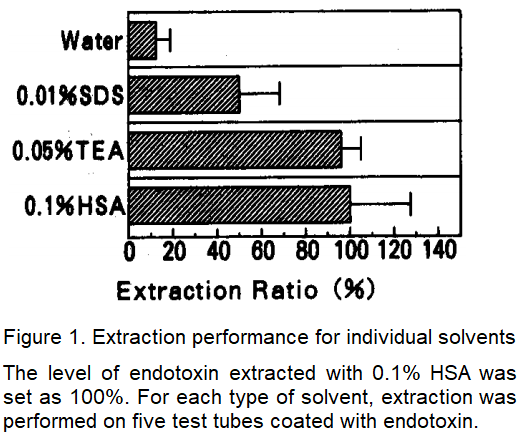
After some consideration, it was determined that the model should involve drying a coat of an endotoxin-containing ethanol suspension applied to polypropylene test tubes, which tend to be less contaminated. Although the addition of ethanol and the process of drying alters endotoxin activity, we were able to create a provisional model in which endotoxin is virtually undetectable when employing water-based extraction.
Extraction performance for water, Sodium Dodecyl Sulfate (SDS), Triethylamine (TEA) and HSA were compared with this model (Figure 1). Concentrations for each solvent were adjusted to levels that would not interfere with LAL testing.
We subsequently learned that endotoxin which could not be completely extracted with water could be extracted efficiently with TEA and HSA. One past study described how SDS was used to extract endotoxin from medical devices2), but our model showed that this solvent is less effective compared to TEA and HSA.
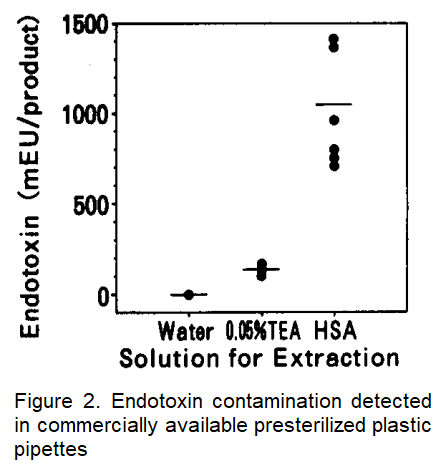
Furthermore, after using water, TEA, and HSA to extract endotoxin from commercially available presterilized disposable plastic pipettes, it was found that HSA was more efficient than TEA at extracting endotoxin (Figure 2).
In other words, when endotoxin contamination among various types of presterilized disposable labware was examined using HSA-based extraction, it was discovered that this method has a better rate of detecting endotoxins from pipettes, pipette tips, serum tubes and cell culture plates. Endotoxin contamination was even observed in some pipette tips that claimed to be guaranteed endotoxin-free.
For this reason, endotoxin contamination in some plastic labware cannot be detected with water extraction. Such undetectable contamination may influence test results, especially when handling proteins. Although the detection method we devised using HSA extraction has not yet been proven applicable for all types of contamination, it can be very effective when compared to routine water extraction procedures. Hopefully this method helps avoid labware contamination in your experiments.
References
- Proceedings of the 6th Annual Meeting of the Japanese Society for Alternatives to Animal Experiments (pp. 110-111). (1992).
- Twohy, C. W. and Duran, A. P. : J. Parenter. Sci. Technol., 40, 287 (1986).
- Roslansky, P. F. et al. : J. Parenter. Sci. Technol., 45, 83 (1991).




