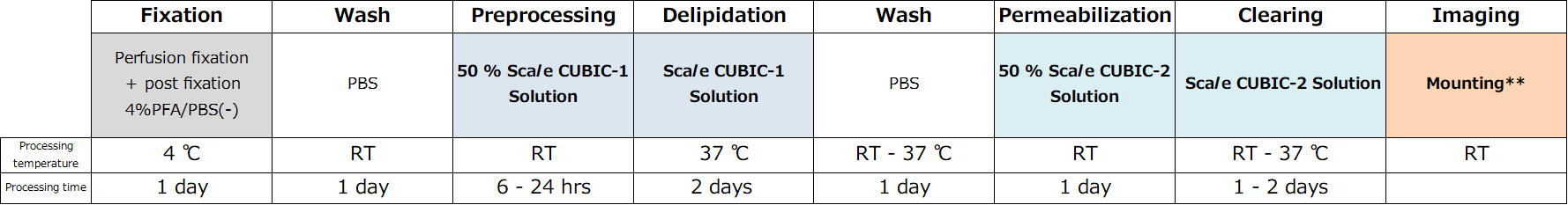
CUBIC
Dr. Hiroki R. Ueda et al. developed a simple, efficient, and scalable brain / body clearing method and imaging / computational analysis pipeline, CUBIC (clear, unobstructed brain/body imaging cocktails and computational analysis). CUBIC offers a fluorescent-preserving, high-performance and device-free tissue clearing method based on hydrophilic Solutions (ScaleCUBIC Solutions).
It enables reproducible whole-organ and whole-body clearing and rapid 3D imaging with LSFM. CUBIC also provides processing and analysis of 3D images for extracting biological information.
Therefore, CUBIC presents a platform of whole-organ/body imaging with single-cell resolution and image informatics, which enables a wide range of users to perform experiments targeting cellular and organ layers with multiple samples..
Data provided by Dr. Etsuo A Susaki, and Dr. Hiroki R. Ueda, RIKEN
Features
- Easy-to-use
- No special equipment required
- Follow up 2D H&E
- High transparency
- Applicable for many organs
- Compatible with IF, FP and other fluorescent labels
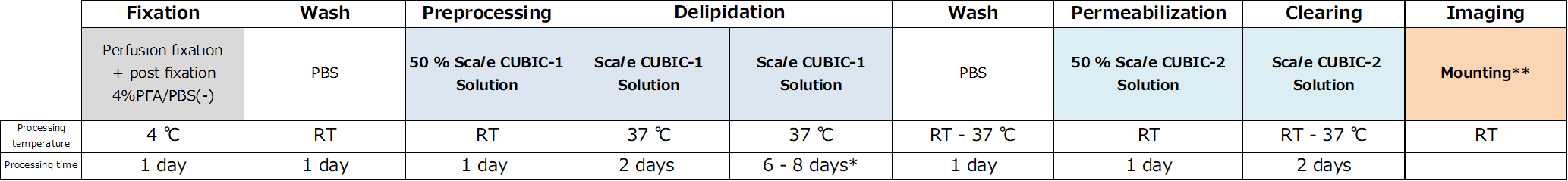
Protocol
Fluorescent protein expression tissue
Whole organ samples
**Typically, 3:7 mixture of Mounting Solution 1 and 2 (RI ~1.49) can be used.
| Brain | Heart | Lung | Kindney | Liver | |
| PBS | 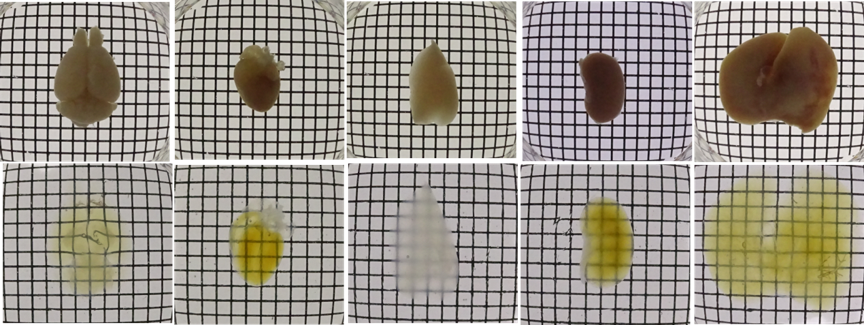 |
||||
| CUBIC | |||||
Figure1.Transmittance images of mouse whole organs before and after clearing with Scale CUBIC Solutions.
2 mm section samples
**Typically, 3:7 or 2:8 mixture of Mounting Solution 1 and 2 (RI ~1.49) can be used
| Brain | Heart | Liver | |
| PBS |  |
||
| CUBIC |  |
||
Figure2.Transmittance images of mouse 2mm slices before and after clearing with Scale CUBIC Solutions.
Fluorescent protein expression tissue

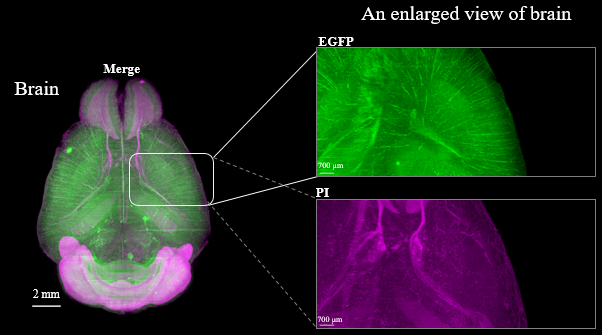
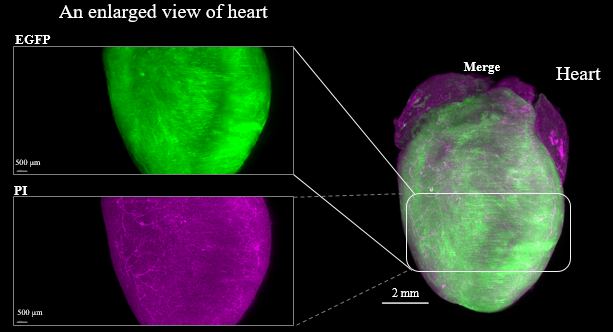
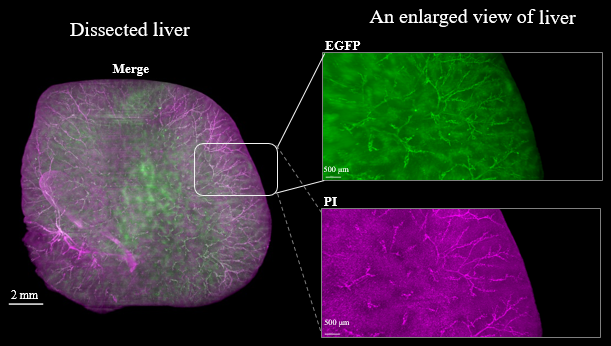
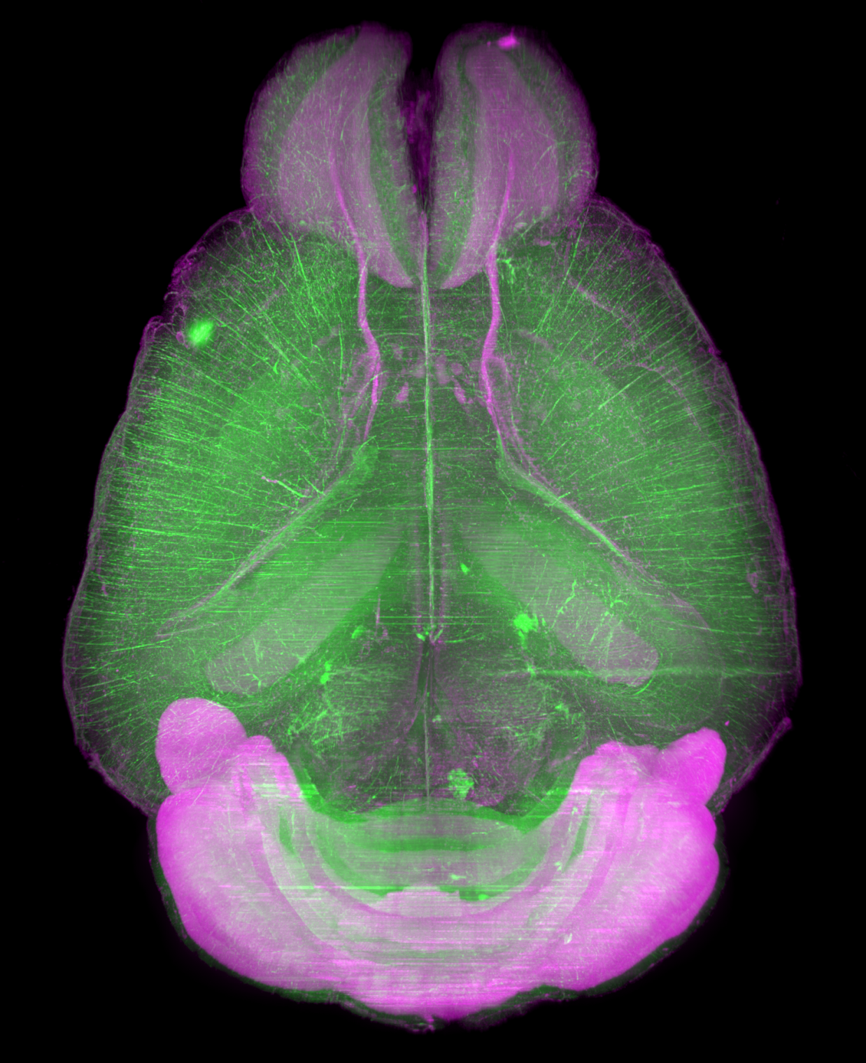
Figure3. LSFM imaging of CAG-EGFP Tg Mouse (8w Male) whole brain, whole heart and dissected liver.
Application
CUBIC Application with pathological methods
CUBIC Application with fish clearing
CUBIC Application with crustacean clearing
CUBIC Application with beetle clearing
Visualization of whole-brain vascular networks
CUBIC Application with pathological methods
Data by S. Nojima.et al. : Scientific Reports ,7,9269 (2017) / Adapted.
|
Before CUBIC |
After CUBIC ------------------------------------------------------------------------------------------- |
||||||
| 0 day | 2 days | 3 days | 4 days | 6 days | 7 days | ||
| Human lung |
CUBIC | 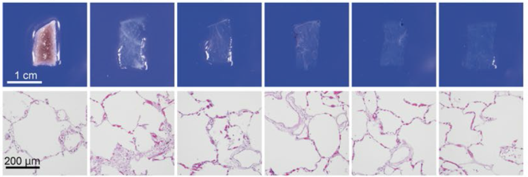 |
|||||
| HE stain | |||||||
| Human Lymph node |
CUBIC | 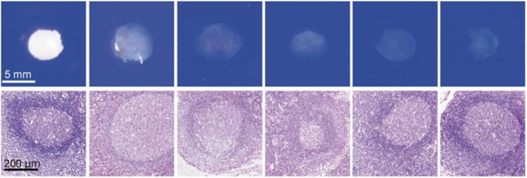 |
|||||
| HE stain | |||||||
Figure 4.Tissue clearing of various human organs with CUBIC
Tissue clearing and 3D imaging of deparaffinizedsamples with CUBIC
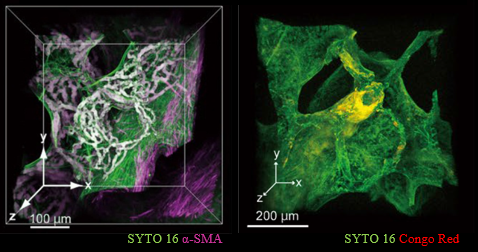
Figure 5.Tissue clearing and 3D imaging of pathological specimens with CUBIC.
CUBIC Application with fish clearing
Data were provided by Kunihiko Futami, Tokyo University of Marine Science and Technology.
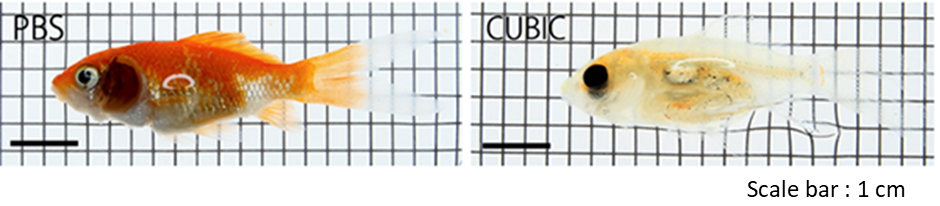
Figure6. Fish clearing with CUBIC
See Reference 5) for the protocol
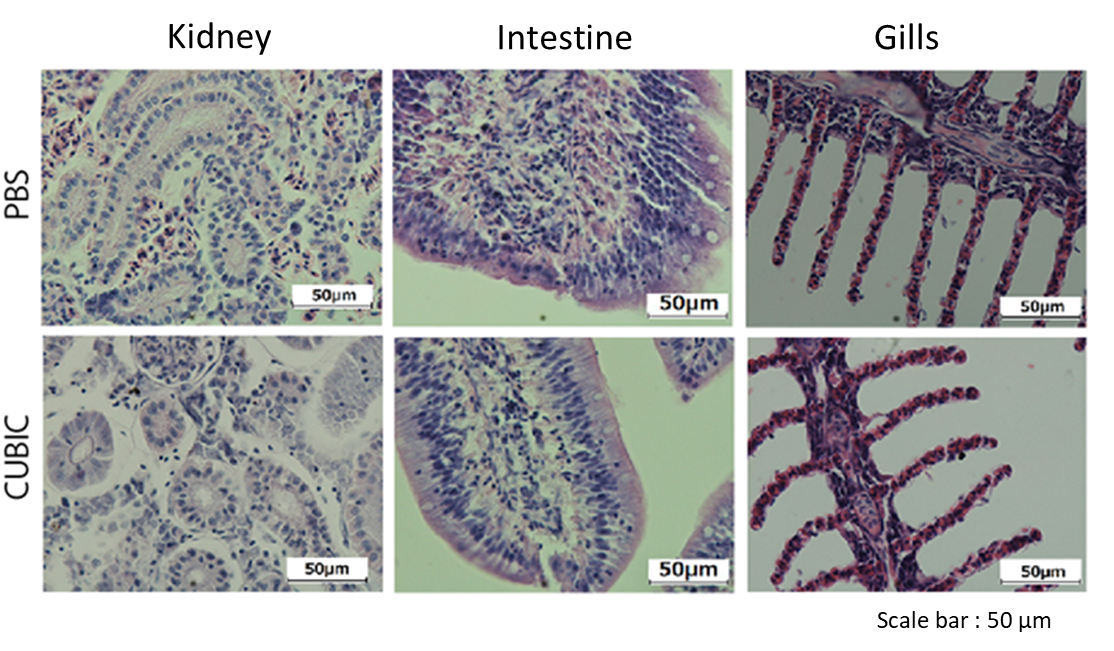
Figure7. Stained image of Fish organ treated by CUBIC
Organs were sliced at 5 μm and stained with Mayer's hematoxylin after CUBIC treatment.
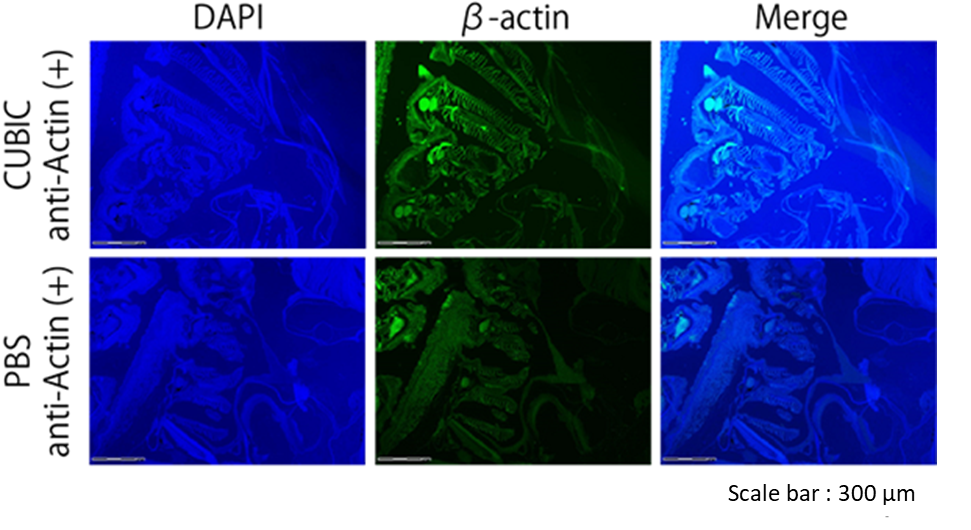
Figure8. Immunostaining image of zebrafish treated with CUBIC
CUBIC Application with crustacean clearing
Data were provided by Aru Konno and Shigetoshi Okazaki, Hamamatsu University School of Medicine in Japan.

Figure9. Crustacean clearing with CUBIC
See Reference 6) for the protocol
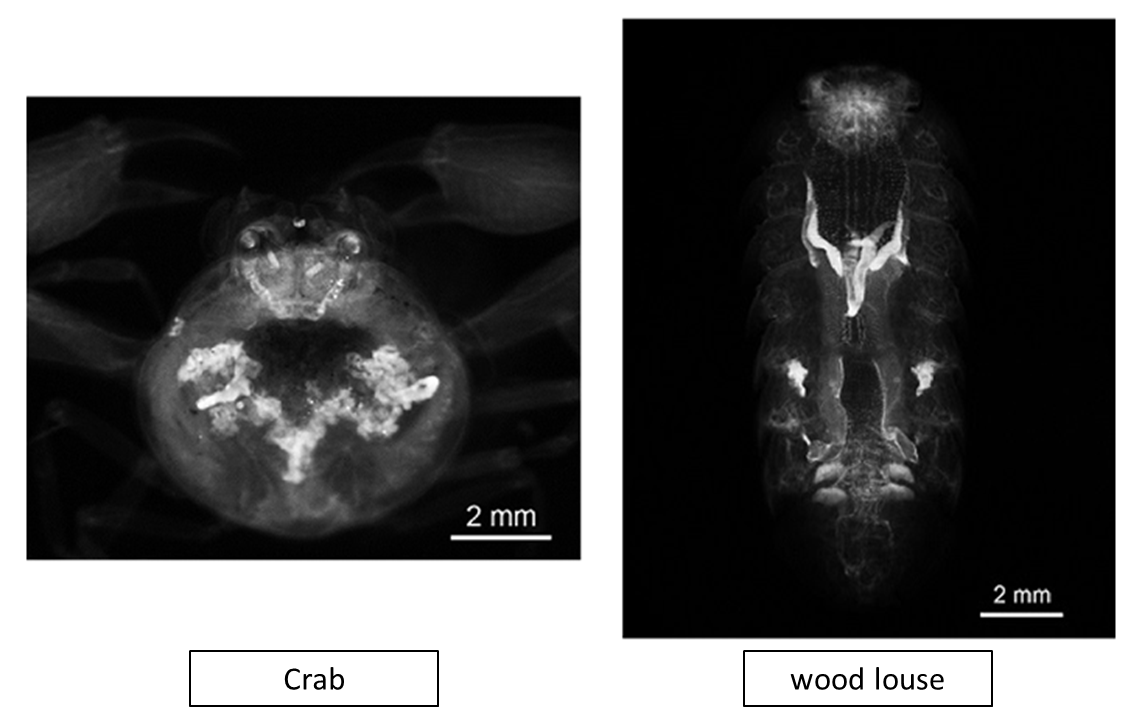
Figure10. Crustacean clearing with CUBIC (PI staining)
See Reference 6) for the protocol
CUBIC Application with beetle clearing
Data were provided by Shinya Kuroda, Tokyo University
Beetle clearing requires fixing, bleaching, and clearing steps.
See the website for Detailed protocols.

Figure11. Beetle clearing with CUBIC
LEFT: drone beetle MIDDLE: rhinoceros beetle RIGHT: stag beetle
This protocol altered from reference 7).
Visualization of whole-brain vascular networks
Data by Miyawaki, T. et al. : Nat. Commun., 11:(1) (2020)
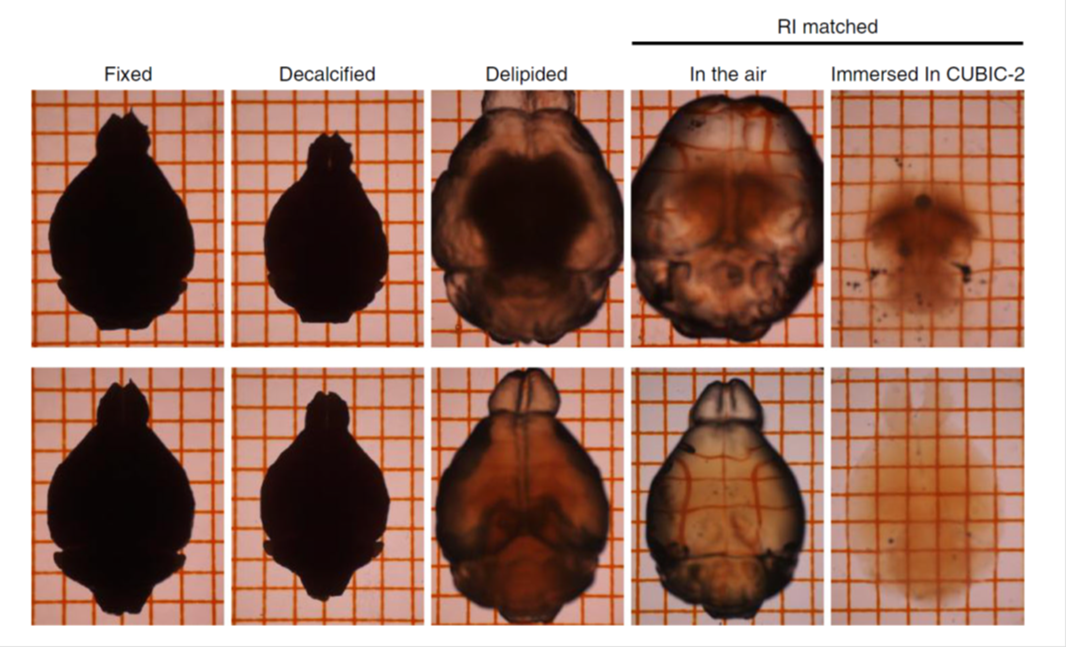
Figure12. Tissue clearing with sodium deoxycholate (SDC)
TOP: SDS-based delipidation. The tissue was swelling.
BOTTOM: SDC-based delipidation. Tissue swelling was suppressed.
This protocol altered from reference 8).
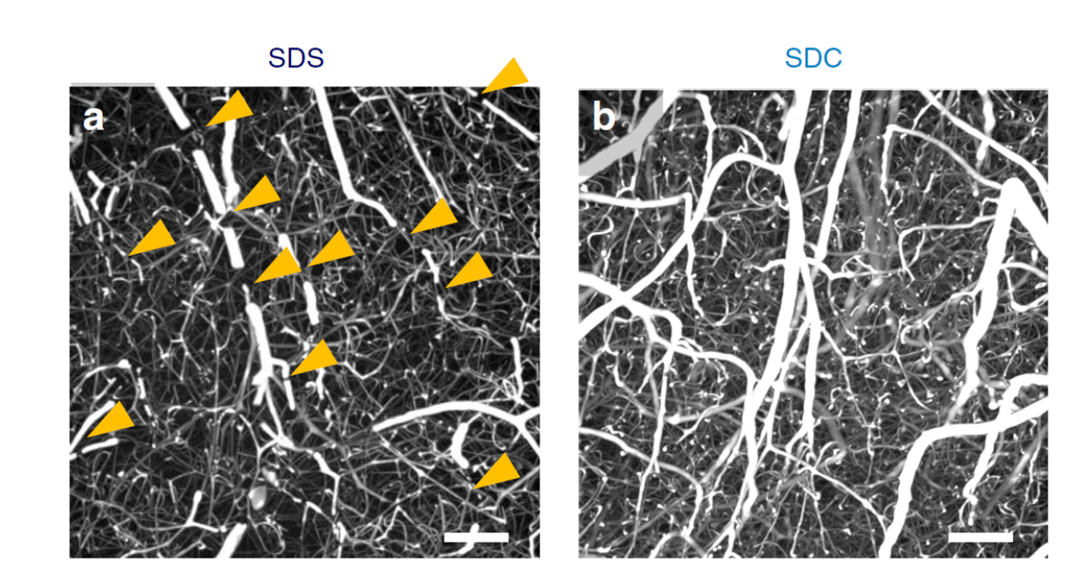
Figure13. Mouse brain cortex clearing after vascular casting
LEFT: SDS-based delipidation. Some penetrating vessels were severed (arrowheads).
BOTTOM: SDC-based delipidation. Breakages of vessels were rarely.
This protocol altered from reference 8).
References
- E. A. Susaki. et al. : Cell, 157(3), 726 (2014).
- K. Tainaka. et al. : Cell, 159(4), 911 (2014).
- E. A. Susaki. et al. : Nature Protocols, 10, 1709 (2015).
- S. Nojima. et al. : Scientific Reports, 7, 9269 (2017).
- S. Ohnuma. et al.: Fish Pathology, 52(2), 96(2017).
- A. Konno and S. Okazaki.: Zoological Letters, 4:13(2018).
- M Kuroda and S Kuroda (2020). Whole-body clearing of beetles by successive treatment of hydrogen peroxide and CUBIC reagents.
- Miyawaki, T. et al. : Nat Commun., 27, 11(2020).
Product List
- Open All
- Close All
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.