Drug Effects
This article was written by Dr. Masakazu Tsuchiya, FUJIFILM Wako Pure Chemical Corporation, for Vol. 60, No. 3 (July 1992) / Vol. 60, No. 4 (October 1992) / Vol. 61, No. 1(January 1993) of Wako Junyaku Jiho.
The content of this article is from the time of publication. It is not the latest information due to new knowledge and changes in regulatory rules after original publication.
Endotoxin measurements of drug products should be carried out under conditions in which the drug does not interfere with the measurement. Here we will look at some methods used to establish conditions for testing drugs, taking into account our actual experience.
Dilution is the most common method used to eliminate sample interference during measurements. Repeated dilution can eventually eliminate interference, but as mentioned in previous episodes, there is a limit on how much a sample may be diluted. For the sake of time, effort, and diluting out the endotoxin in a sample, it is beneficial to use as small of a dilution as possible.
Therefore, the sample concentration at which there is no interference must be found first in order to decide measurement conditions for a sample. The United States Pharmacopoeia1) and FDA Guidelines2) provide useful information on how to determine the sample concentration that eliminates interference. According to these guidelines for the gel-clot test, a sample is said to have no effect when the end point of the endotoxin solution diluted with the sample solution is within 1/2 to 2 times of the labeled sensitivity of the Limulus reagent. For the kinetic turbidimetric assay and the synthetic substrate method, a sample is said to have no effect when the recovery is 100±25% for a sample spiked with 4 times the smallest concentration for the standard curve.
Recently, the tentative Guidelines3) released by the FDA have changed the permissible spike recovery range to 100±50% for the kinetic turbidimetric assay. The Japanese Pharmacopoeia does not set standards for the effects of samples, and therefore results have to be analyzed based on the objectives for each study. We have set an endotoxin recovery range of 100±25% at the stage of examining measurement conditions.
Now, let's take a look at the actual procedure. Endotoxin is first added to each sample concentration. The spike method can be carried out by adding the endotoxin solution to different concentrations of sample solutions, or by diluting the endotoxin spiked sample solution with endotoxin solution of the same concentration. The method needs to be selected based on the nature of the sample and the concentration required for measurement.
Here we will give an example of how to test a sodium citrate injection solution using the kinetic turbidimetric assay.
Each concentration (0.06% - 2%) of sodium citrate injection solution (4 mL) received 20µL of a 12.5EU/mL endotoxin solution. The sample was measured with Limulus HS-J Test Wako as the Limulus reagent, and the Toxinometer® ET-201 was used as the measuring device. The sample solution without added endotoxin was also measured.
A 4 point endotoxin standard curve was prepared from a 2-fold dilution series spanning 0.0156 to 0.125 EU/mL. A 0.0625 EU/mL spike was added to each sample concentration, which is 4 times the minimum endotoxin concentration(λ)on the standard curve.
The endotoxin concentration for each diluted sample was calculated from the gelation time (Tg) of the sample, and a 100% spike recovery was assumed to occur in samples with a calculated concentration of 0.0625 EU/mL. The relationship between sodium citrate concentration and endotoxin recovery rate is shown in Fig.1. Samples which did not have any added endotoxin showed no gelation. You can see that sodium citrate inhibits measurements at 2%, but inhibition decreases with dilution. In this test sample, the endotoxin recovery rate reached 75% or more at 0.125%, making measurements with this concentration valid.
Based on results obtained from these studies, measurement conditions can be determined in consideration of the regulated endotoxin detection limit and MVD. The effect of the drug on a measurement can vary depending on the product being tested. This may be due to differences in manufacturing methods and coexisting materials. Also, caution must be taken as each measurement method (kinetic turbidimetric assay, gel-clot method, and synthetic substrate method) has its own unique features and may be affected differently.
At the time of this study, water for Injection was the only drug for which the Japanese Pharmacopoeia required endotoxin testing. However, now the bacterial endotoxins test is being applied to other drugs, and studies are performed by a public-private joint project of the Japan Health Science Foundation. In the future, the test is expected to be applied to various other drugs, as it is in the United States.
The drugs that will be tested using the bacterial endotoxins test are those that are currently being tested using the rabbit pyrogen test. Here we will investigate the concentration at which the drug affect the bacterial endotoxin test. We tested various drugs for which the Japanese Pharmacopoeia requires pyrogen testing.
Our investigation was carried out in the same way as the study on sodium citrate presented in Episode 8. We used Limulus HS-J test Wako as the Limulus reagent, and the Toxinometer® ET-201 as the testing device. For this investigation we determined that there was no sample interference if the recovery rate of endotoxin was 100±25%.
Figure 1 shows the drugs that were measured, concentrations at which the recovery rates were acceptable, and the MVD of each drug.
| Name of drug | 100±25% recovery rate was obtained | MVD* | |
|---|---|---|---|
| Sample drug concentration | (dilution ratio) | ||
| Meglumine Amidotrizoate Injection (65%) | 0.81% or less | (80 fold or more) | 80 fold● |
| Meglumine Sodium Amidotrizoate Injection(76%) | 0.95% or less | (80 fold or more) | 94 fold● |
| Sodium Iotalamate Injection(80%) | 1.0% or less | (80 fold or more) | 123 fold● |
| Meglumine Iotalamate Injection(60%) | 1.5% or less | (40 fold or more) | 160 fold |
| Meglumine Iodamide Injection (64.9%) | 0.81% or less | (80 fold or more) | 80 fold● |
| Meglumine Sodium Iodamide Injection (80%) | 2.0% or less | (40 fold or more) | 106 fold● |
| Isotonic Sodium Chloride(0.9%) | 0.9% or less | (1 fold or more) | 32 fold |
| Magnesium Sulfate Injection(500mM) | 100mM or less | (5 fold or more) | 712 fold |
| Sodium Bicarbonate Injection(7%) | 0.5% or less | (14 fold or more) | 266 fold |
| Ringer's solution ** | Undiluted solution | (1 fold or more) | 32 fold |
| Sodium Citrate Injection for Transfusion (10%) | 0.125% or less | (80 fold or more) | 128 fold |
| Sulfobromophthalein Sodium Injection(5%) | 0.00125% or less | (4000 fold or more) | 205 fold |
| Heparin Sodium Injection(1000U/mL) | 0.01 U/mL or less | (105fold or more) | 160 fold● |
| Dehydrocholic Acid Injection (20%) | 0.25% or less | (80 fold or more) | 512 fold |
| Mannitol(20%) | 5.0% or less | (4 fold or more) | 32 fold |
| Dextran40(10%) | 2.5% or less | (4 fold or more) | 64 fold |
| Dextran70(6%) | 0.75% or less | (8 fold or more) | 32 fold |
| Fructose injection(20%) | 1.25% or less | (16 fold or more) | 32 fold |
| Glucose injection(50%) | 2.5% or less | (20 fold or more) | 320 fold |
| Xylitol Injection(20%) | 2.5% or less | (8 fold or more) | 128 fold● |
Figure 1 Effects of each drug on measurement
* The MVD calculations were based on the endotoxin limits set forth in FDA Guidelines. Values with "●" are for drugs which did not have preestablished limits, and the MVD was calculated from the dose given to rabbits in the pyrogen test for the Japanese Pharmacopoeia. The value of λ was set at 0.0156 EU/mL.
** Ringer's Solution Composition:0.86% NaCl, 0.03% KCl, 0.033%CaCl2
Various contrast media (amidotrizoate, iothalamate, and iodamide), inorganic salts, and sugars showed endotoxin recovery rates of 100±25% within the MVD, without impacting the reaction mixture's pH or the reaction time course.
Sulfobromophthalein sodium is colorless in acidic solutions, but turns deep purple in neutral or alkaline solutions. It was found to turn purple when it reacted with LAL and a white sediment was observed when a sample concentration of 0.05% or more was mixed with LAL. This sediment was no longer observed at a concentration of 0.005% (1,000 fold dilution) or less, and no effects on endotoxin recovery were observed at 0.00125% (4,000 fold dilution) or less.
The minimum concentration of the calibration curve for the measurement in this study was 0.0156EU/mL, so the MVD was 3,205 fold. Lowering the minimum concentration of the calibration curve to 0.0078 EU/mL (gelation time around 60 minutes) multiplied the MVD to 6,410 fold, which enabled measurement of the drug.
Heparin Sodium showed enhancement effects at high concentrations, and the concentration at which endotoxin recovery rate was 125% or less was 0.01 U/mL (105 fold dilution). However, the recovery rate was around 150% at a 10 fold dilution, thus the gel-clot method was thought to be a possible measurement method. Under the gel-clot method, measurement was possible within the MVD (assuming gel-clot sensitivity of λ = 0.03 EU/mL, 80 fold) since a drug is assumed to have no effect on the measurement when the gel-clot endpoint of the spiked sample is found to be within ±2 λ.
According to the tentative guidance 4) issued by the FDA recently, an endotoxin recovery rate of 100±50% is an allowable range for the kinetic turbidimetric assay method. If this guidance is followed, the sample in this study could also be measured with the kinetic turbidimetric assay using a dilution within the MVD.
The results of this study show that using specific measurement conditions allows for the application of the Limulus test to most of the drugs that were examined. We believe it is possible to replace the rabbit pyrogen test with the Limulus test in the future, and we would like to hear opinions from others on this matter in the future.
As mentioned above, the drug effects can be categorized into the following three types:
- Effect on the activity of the Limulus Amebocyte Lysate (LAL) reagent
- Effect on the physical properties of endotoxin
- Effect on the measurement in ways other than the main reaction
Many of the effects observed from drugs are thought to be due to No. 1 above, where the drug affects the enzymes in the LAL reagent. However, some drugs clearly affect the physical properties of endotoxin, which causes problems for validation of the measurement. For example, if the drug is known to have no effect on the LAL enzyme system, but the endotoxin recovery rate is still not good, the drug may be interfering with the endotoxin's physical properties. Let's take a look at the effect described in 2.
We have observed a case where metal ions such as iron and aluminum affected only the activity of endotoxin5). The results indicated that the metal salt solution lowered the activity of endotoxin at concentrations around 10 µM but had no effect on the LAL enzyme system. We will explain the experimental methods below.
In a normal spike recovery test for endotoxins, endotoxin is added to the sample and then mixed with LAL to carry out the measurement. When a spike recovery test was carried out for 40 µM of iron salt and 20 µM of aluminum salt, both had a recovery rate of 30% or less (Table 2).
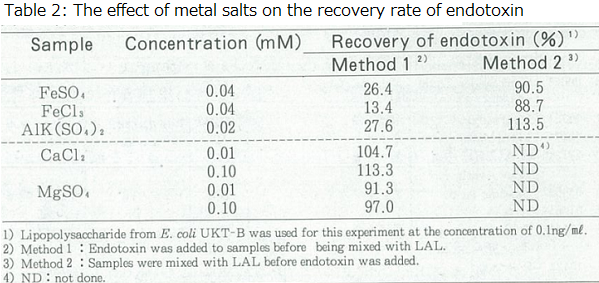
Since we did not think it was plausible for low concentrations (µM) of metal ions to inhibit the LAL enzymes, we conducted the following experiment. The metal salt solution was first mixed with the LAL, and then the endotoxin solution was added to the mixture. If the metal ions influenced the LAL enzymes, the effects would be observed under both methods for preparing the reaction mixture.
However, the recovery rate for endotoxin was observed at 88-114%, with no effect on the measurement (Table 2). The difference in these experiments was whether the endotoxin made contact with the sample or the LAL first. Of course, mixing the sample with the LAL lowered the concentration of the sample, and it might have eliminated the effect of the sample on the endotoxin; however, in practice, a few µM of metal salt solution lowered the activity of endotoxins, therefore this view gained no support.
It seems that this phenomenon can be explained if we assume that the sample affects endotoxin and somehow changes its activity but it does not affect the LAL enzyme system. If this is the case, the endotoxin must be more reactive with the LAL than the metal ion. This implies that proteins moderate the effect of metal ions on the endotoxin molecule, and that the affinity between Factor C (Endotoxin-sensitive substances) in LAL and endotoxin is extremely strong.
This method cannot detect all of the substances that have an effect on endotoxin itself. However, in the case where an effect is observed only when the sample is mixed with endotoxin first, we can presume that the sample has some kind of effect on endotoxin itself.
In cases where an endotoxin standard is used, caution must be given to mixing it with any substances that have an effect on endotoxin. The alteration of activity in an endotoxin standard solution could obstruct measurements. We have also observed that extracts from soda lime glass could lower the activity of endotoxin standards. In addition, a decline in endotoxin activity was observed when a metal instrument was immersed in an endotoxin solution for a long time.
Meanwhile, it seems that naturally occurring endotoxin in a sample that affects endotoxin activity already exists in the affected state. The activity might not be further affected by changing conditions, but because the activity of purified endotoxin can be easily modified by ultrasonic treatments or freeze drying, it is possible that additional changes to endotoxin activity can be made.
Therefore, we need to consider the benchmark conditions for standard activity. This is a difficult question, with no simple answer. For now, standardization needs to be tailored to the objective of the measurement.
For method validation, it is not necessary to know the exact type of effect caused by the sample. The effect of the sample can be minimized with dilution and measurement is possible if the specified criteria are met, regardless of which type of effect the sample may cause. However, we believe it would be easier to establish the measurement conditions if we understood the types of effects samples may have.
References
- The United States Pharmacopeia 22th, The National Formulary 17th, p.1493-1495, Pharmacopeial Convention Inc., MD (1989).
- Guideline on validation of the Limulus amebocyte lysate test as an end-product endotoxin test for human and animal parenteral drugs, biological products, and medical devices, Food and Drug Adm. (1987).
- Interim guidance for human and veterinary drug products and biologicals, Food and Drug Adm. (1991)
- Interim guidance for human and veterinary drug products and biologicals, Food and Drug Adm. (1991)
- Masakazu Tsuchiya: Journal of antibacterial and antifungal agents, 18, 287-294 (1990).




