β-glucan
This article was written by Dr. Masakazu Tsuchiya, FUJIFILM Wako Pure Chemical Corporation, for Vol. 61, No. 2 (April 1993) / Vol. 61, No. 3 (July 1993) of Wako Junyaku Jiho.
The content of this article is from the time of publication. It is not the latest information due to new knowledge and changes in regulatory rules after original publication.
LAL reacts not only to endotoxin, but also to (1→3)- β-D-glucan to form a gel. This phenomenon was reported by two Japanese groups in 1981. The first, Dr. Kakinuma from Takeda Pharmaceutical Company, reported that 1 to 1000ng/mL of carboxylmethylated curdlan (linear (1→3)-β-D-glucan produced by agrobacterium) activates the proclotting enzyme and causes a gelation of the Limulus reagent (LAL)1). The second, Prof. Iwanaga's group from the University of Kyushu, similarly reported that a gelation system triggered by (1→3)-β-D-glucan exists in LAL2).
At the "International Conference on Endotoxin Standards and Limulus Amebocyte Lysate Use With Parenteral Drugs" hosted the same year in Woods Hole, Massachusetts (USA), two topics were presented3,4) regarding LAL reactive materials(LAL-RM)without any pyrogenic properties. These LAL-RMs are eluted from cellulose type membranes that are used with hemodialysis equipment. It was also reported that reactivity of the LAL-RM changes depending on the manufacturer of the LAL reagent.
However, even after the presentations from two US group, scientists in the EU and US could not break from their view that the substance reacting with LAL is endotoxin, and they could not come to terms with the fact that β-glucan could cause activation of LAL.
Today, β-glucan's role in triggering activation of LAL is widely acknowledged, and the United States FDA has also recognized that LAL-RM and glucan can both react with LAL in its Memorandum5) published in May 1992. However the memorandum also said that "the only product that has been shown to contain LAL-RM is dialysis membranes made of cellulose...No cases of glucans or LAL-RM have been reported in parenteral drug products...the presence of glucan in parenteral drug products and most medical devices [is] more of a theoretical than actual problem." The memorandum did note that the FDA would consider glucan contamination on a case-by-case basis if there are problems.
Well-known examples of glucans which react to LAL include curdlan, LAL-RM, Zymosan and Lentinan produced by fungi, and Laminarin used as an energy reserve compound in algae. Also, some cellulose-type membrane filters elute substances similar to LAL-RM.
We have experienced that some parenteral drugs and their raw materials(e.g. various amino acids and blood products)are contaminated with glucans6). Caution must be taken in light of this fact if appropriate results are to be obtained from the Limulus test.
LAL reactivity to β-glucan depends on the LAL manufacturer. Comments made by LAL-RM researchers point to the fact that LAL-RM reacts well to LAL manufactured by Associates of Cape Cod, but not at all to LAL manufactured by Whittaker (now Lonza) .
In addition, Pregel-M (Manufactured by Teikoku Hormone and distributed by Seikagaku Corporation), Pyrosate (manufactured by Hemachem and distributed by Green Cross Corporation)and Limulus HS-J and HS-F Test Wako (manufactured by Endosafe (now Charls River Labs)) react to β-glucan to varying degrees.
For synthetic substrate method LAL, Toxicolor(Seikagaku Corporation)reacts to β-glucan, but CQL1000(manufactured by Whittaker (now Lonza), distributed by Daiichi Kagaku)shows less reactivity.
The reactivity of Limulus Amebocyte Lysate (LAL) to (1→3)-β-D-glucan can be studied through various methods, including those based on structure, concentration dependence, and reaction kinetics. In terms of structure, a 13C-NMR study found that activation of the β-glucan-sensitive factor (Factor G) in LAL was 100 times more potent with single helix conformations compared to triple helix conformations7).
In terms of concentration dependence, it has been reported that the optimal concentration range for reactions is 100 to 1000 ng/ml, and that LAL activation does not occur at higher concentrations 8). Furthermore, we described in another study the reaction kinetics of LAL with (1→3)-β-D-glucan examined using the Toxinometer® ET-201 (FUJIFILM Wako Pure Chemical Corporation)9).
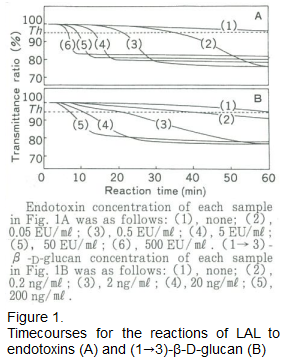
This post delves into the reaction kinetics of LAL towards (1→3)-β-D-glucan.
After analyzing the reaction time course curve from LAL activation, we discovered that LAL reacts differently with endotoxins and β-D-glucan. The reaction of LAL with endotoxin shows a long time lag followed by a rapid change in turbidity due to gelation, while the reaction with (1→3)-β-D-glucan shows a short time lag followed by a more gradual change in turbidity (Figure 1).
With a mixture of endotoxin and (1→3)-β-D-glucan, the reaction displays a combination of both characteristics - a short time lag then sudden change in turbidity. This disparity in reactions to endotoxin and (1→3)-β-D-glucan is believed to be related to different activation pathways for Factor C (an endotoxin-sensitive factor) and Factor G within LAL.
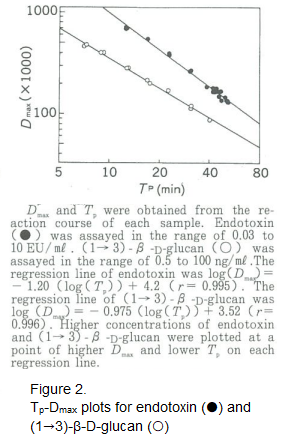
In order to quantitatively evaluate this difference, we concentrated our efforts on using the differential coefficient of the reaction time course curve. The ME laboratory at FUJIFILM Wako Pure Chemical Corporation created a computer program to calculate these differential coefficients. The maximum values of the differential coefficients determined by the program were designated as Dmax and the reaction times to Dmax was designated as Tp. When Dmax and Tp data for endotoxins and (1→3)-β-D-glucan were plotted on a graph, they formed distinct linear slopes (Figure 2).
We called this process the Tp-Dmax plot method and tried to use it to distinguish reactions of endotoxins from those of β-glucans. However, plans for practical applications were abandoned because the necessity for post-measurement analysis raised issues with respect to quantitative performance and general versatility.
Nevertheless, even without Tp-Dmax plots, it is possible to eventually determine whether endotoxin or β-glucan is the primary substance activating LAL by becoming familiar with reaction time courses. In this sense, it can be said that observing reaction time courses with the kinetic turbidimetric assay is a meaningful endeavor because this kind of information cannot be obtained with the gel-clot method or end point synthetic substrate method.
There is much more room for further research into the reaction of LAL with (1→3)-β-D-glucan as compared to the reaction with endotoxins. Specifically, it would be interesting to study how LAL reacts to substances with multiple types of bonds and sugars.
References
- Kakinuma, A. et al.: Biochem. Biophys. Res. Commun., 101, 434 (1981).
- Morita, T. et al.: FEBS Lett., 129, 318 (1981).
- Carson, L. A. and Peterson, N. J.: "Endotoxins and Their Detection with the Limulus Amebocyte Lysate Test," ed. by Watson, S. et al., Alan R.Liss. Inc., New York, p.217 (1982).
- Pearson, F. C. et al.: "Endotoxins and Their Detection with the Limulus Amebocyte Lysate Test," ed. by Watson, S. et al., Alan R. Liss. Inc., New York, p.247 (1982).
- Statement Concerning Glucans and LAL-Reactive Material in Pharmaceuticals and Medical Devices, Food and Drug Adm. (1992).
- Masakazu Tsuchiya: The Society for Antibacterial and Antifungal Agents, Japan 18, 287(1990).
- Saito, H. et al. : Carbohydr. Res., 217, 181 (1991).
- Kakinuma, A. et al. : Biochem. Biophys. Res. Commun., 101, 434 (1981).
- Tsuchiya, M. et al. : Chem. Pharm. Bull., 38, 2523 (1990).




