Limulus Amebocyte Lysate (LAL) and Silkworm Larvae Plasma (SLP) Reagents
This article was written by Dr. Masakazu Tsuchiya, FUJIFILM Wako Pure Chemical Corporation, for Vol. 63, No. 1 (January 1995) of Wako Junyaku Jiho.
The content is from the time of the presentation. It is not the latest information due to new knowledge and changes in regulatory rules after original publication.
Limulus amebocyte lysate (LAL) reagent reacts with endotoxin and (1→3)-β-D-glucan (β-glucan), which make up the cell walls of gram-negative bacteria and fungi. Our research has focused on the detection of the components of microbial cell walls. In particular, we focused on peptidoglycan (PG), endotoxin, and β-glucan as the markers of microbial cell walls.
PG constitutes the cell wall of bacteria and has a variety of biological activities. PG may be useful as a general marker of bacterial cells as it is a component of both gram-positive and gram-negative bacteria. We have previously developed a reagent called the silkworm larvae plasma (SLP) reagent, which can be used to detect both PG and β-glucan.
The SLP reagent was designed based on the prophenoloxidase (ProPO) cascade contained in the body fluid of silkworms. The laboratory of Prof. Masaaki Ashida in Hokkaido University performed extensive studies on the ProPO cascade. Although some details remain to be elucidated, the ProPO cascade is known as a reaction system that is comparable to the mechanism of the LAL gelation cascade.
The ProPO cascade plays an important role in the biological defense mechanism of insects. Figure 1 illustrates the reaction mechanisms for LAL and SLP. Studies demonstrated that β-glucan recognition protein (BGRP) and PG recognition protein (PGRP), which have already been purified from the body fluid of silkworms 1), trigger the ProPO cascade.
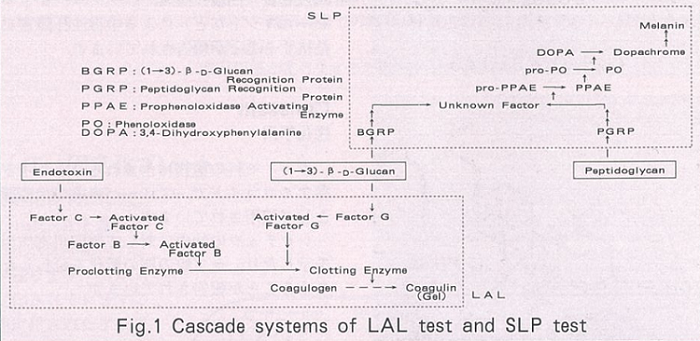
Although the detailed reactions following these initial steps have not been fully elucidated, it is known that phenoloxidase (PO) is activated at the end of the cascade and oxidizes substrates such as L-3,4-dihydroxyphenylalanine (L-DOPA) to form a melanin pigment.
The procedures involved in the measurement of PG and β-glucan using the SLP reagent are similar to those of the kinetic-turbidimetric assay in the LAL test. Specifically, the SLP reagent uses L-DOPA as the substrate of PO and measures the amount of melanin produced by the reaction as the light transmittance ratio of the reaction solution. The amounts of PG and β-glucan are then measured based on the time required until a certain level of decrease in the light transmittance ratio.
The Toxinometer (FUJIFILM Wako Pure Chemical Corporation) and microplate reader M-Tmax (Molecular Devices) are used for SLP assays as well as endotoxin measurements. Melanin formation can also be assessed by visually observing the reaction using the microplate. The latter method is technically feasible and may be widely applicable.
PG forms a thick layer in the outermost layer of the gram-positive bacterial cell wall. However, in gram-negative bacteria, it exists as a thin inner layer of the cell wall below the outer membrane. Considering the amount and location of PG, gram-negative bacteria may not react to the SLP reagent.
Using dried bacterial cells, we studied the reactivity of bacterial species to the LAL and SLP reagents 2). In this study, we demonstrated that as with gram-positive bacteria, most gram-negative bacteria reacted to the SLP reagent. However, there may have been some damage to the bacteria since we used dried cells, and this may in turn have caused the exposure of PG to the outer membrane, inducing a reaction with the SLP reagent. Nevertheless, our findings indicated that the reactivity of gram-negative bacteria to the SLP reagent was equivalent to that of gram-positive bacteria.
The current SLP reagent is not specific to PG, as it also reacts with β-glucan. It has also been reported that the reactivity of SLP to β-glucan is not equivalent to that of the LAL reagent to β-glucan.
While we still do not know how useful the SLP reagent may be, we anticipate that it would inform us of the presence of microbes when used in combination with the pioneering LAL reagent. Future studies will further investigate the nature of the SLP reagent with the aim of developing reagents that are specific to PG and β-glucan.
References
- Masaaki Ashida. KAGAKU TO SEIBUTSU, 26, 425 (1988).
- Fukiko Kobayashi et al. Proceedings of Bioscience, Biotechnology, & Biochemistry, 68, 422 (1994).




