Pyrosep™ Method
This article was written by Dr. Masakazu Tsuchiya, FUJIFILM Wako Pure Chemical Corporation, for Vol. 63, No. 3 (July 1995) of Wako Junyaku Jiho.
The content of this article is from the time of publication. It is not the latest information due to new knowledge and changes in regulatory rules after original publication.
PyrosepTM is a material which contains histidine molecules bound to an appropriate carrier via spacer molecules and is used mainly in the process of endotoxin removal. PyrosepTM (manufactured by Tanabe Seiyaku Co. Ltd., sold by Daicel Corp. and FUJIFILM Wako Pure Chemical Corp.) is sold as an affinity adsorbent for endotoxin and can be used to effectively remove endotoxin under certain conditions using specialized equipment such as capillary columns.
There are two types of PyrosepTM systems: one uses agarose as the carrier (PyrosepTM A), while the other uses cellulose (PyrosepTM C). When compared with PyrosepTM A, PyrosepTM C can be used under a wider range of conditions to effectively adsorb endotoxin.
The degree of endotoxin adsorption can be varied under different conditions when using PyrosepTM A. This property enabled Tanabe Seiyaku to develop a method to measure endotoxin in a sample by selectively adsorbing endotoxin1). In this method, endotoxin in the sample is adsorbed to PyrosepTM and then the remainder of the sample is removed by washing. Adsorbed endotoxin then activates the LAL reagent under alkaline conditions, which allow for more effective endotoxin release.
The PyrosepTM method has the following characteristics:
- Since the sample is removed by washing, the influence of the sample on the LAL reaction can be avoided.
- Sensitivity can be improved with proper experimental design since endotoxin in a sample can be concentrated.
The PyrosepTM method can be performed with a filter cup or a capillary column.
In the filter cup method, a container such as a microplate well with an attached hydrophobic membrane filter is used to mix a sample with PyrosepTM. Endotoxin in the sample is adsorbed to PyrosepTM and then the sample is removed by vacuum filtration. Approximately 20 mM of saline is then added to the cup to remove traces of the sample, followed by further sample removal by vacuum filtration. A chromogenic LAL reagent can then be added to the filter cup to start the reaction with the remaining endotoxin molecules bound to PyrosepTM. When the chromogenic LAL reaction is terminated at the appropriate time, the reaction solution is vacuum filtered one last time and then the absorbance of the filtrate is measured by spectrophotometric methods to determine the amount of endotoxin captured from the sample.
In the capillary column method, a syringe with silicone tubing is attached to a glass capillary tube, which contains a filter made from a material such as glass wool, to prevent PyrosepTM from entering the syringe. The syringe is used to suction PyrosepTM into the capillary tube to create an adsorption column. The sample is suctioned into the capillary tube next. A saline solution is then suctioned up and dispensed to wash out the sample while the endotoxin molecules remain bound to the column. Lastly the LAL reagent is suctioned into the tube halfway and then dispensed along with PyrosepTM to react with the captured endotoxin molecules.
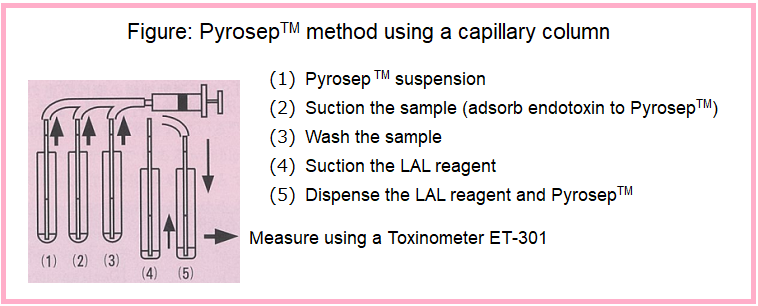
We applied the capillary column method using a Toxinometer® to demonstrate its use. In this study, the sample was diluted with acetate buffer (pH 5.0) and was washed with 2 mL of 20 mM saline in a capillary column. When water was used as a sample, there was no need to dilute it with buffer or to wash it with saline.
The LAL reagent (Limulus ES-II Test Wako) was dissolved to half of its original concentration using 0.2M Tris-HCl buffer (pH 8.0). A total of 0.3 mL of the diluted LAL reagent was suctioned halfway into the capillary column and then dispensed into a reaction tube. A Toxinometer® was used to measure endotoxin in the reaction solution. Using 4 mL of water as the sample, 0.5 EU/L (0.0005 EU/mL) of endotoxin was detected within 60 minutes.
The detection sensitivity we achieved using the method above is approximately 10 times greater than that of a conventional method using a Toxinometer®. With the capillary column method, all endotoxin in a sample can be adsorbed to PyrosepTM and become highly concentrated onto the column when a large amount of the sample is used. This property may be useful when applying the LAL test in settings that require a highly sensitive method of detection.
Filter cups and capillary columns used for the PyrosepTM method must be free of endotoxin. However, it is challenging to manufacture such instruments and the procedure for quantification can be complicated. Nevertheless, the PyrosepTM method may be useful for certain applications since it can be used to carry out reactions under uniform conditions and can concentrate endotoxin from a sample. We aim to further investigate the possible applications of the method, including automation of the steps involved.
Reference
- Minobe, S. et al. : Anal. Biochem., 198, 292-297 (1991).




