Specificity of LAL
This article was written by Dr. Masakazu Tsuchiya, FUJIFILM Wako Pure Chemical Corporation, for No. 4 (March 1991) of Wako Junyaku Jiho.
The content of this article is from the time of publication. It is not the latest information due to new knowledge and changes in regulatory rules after original publication.
Specificity of LAL
Limulus Amebocyte Lysate (LAL) reacts to both endotoxin and (1→3)-β-D-glucan. In 1981, Kakinuma et al. reported the reaction of carboxymethylated (1→3)-β-D-glucan (CMPS) to LAL1), and in the same year, Morita et al. reported that (1→3)-β-D-glucan activated LAL via another pathway, which was different from that of endotoxin2).
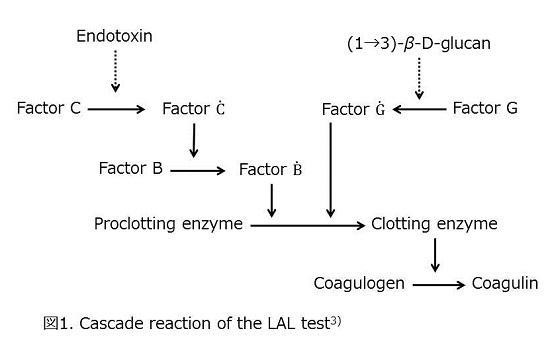
Iwanaga et al. clarified the LAL reaction mechanism, as shown in Figure 13). In addition, Pearson et al. reported the elution of nonpyrogenic LAL reactive matter (LAL-RM) from a hemodialysis device and mentioned that this could be β-glucan4).
Söderhäll et al. confirmed that CMPS reacted to LAL, but also reported that natural β-glucan did not react to LAL based on an experiment using laminarin and polymyxin B5). Hodes et al. investigated the reactivity of the LAL reagent from Japanese horseshoe crabs (Tachypleus Amebocyte Lysate, TAL) and US-manufactured LAL to fungus extracts and reported that TAL reacted to fungus extracts while LAL did not6). In addition, Bayston and Cohen mentioned that false positive reactions can occur, but false positive substances are rare in clinical samples and the main cause of contamination is endotoxin7).
There seems to be a contradictory view overseas regarding the reactivity of LAL to β-glucan, though this reactivity has mostly been accepted in Japan. This is because the Limulus test is mainly used in the semi-quantitative gel-clot method overseas, and some LAL products on the market have a low reactivity to β-glucan.
By analyzing the LAL activation reaction time course with the Toxinometer measurement system (FUJIFILM Wako Pure Chemical Corporation), we demonstrated that endotoxin and β-glucan have different reactions with LAL8). While the reaction lag was long and changes occurred suddenly in the reaction time course of the LAL-endotoxin reaction, the LAL-β-glucan reaction showed a more gradual change despite a short reaction lag.
It was also observed that the reaction time course of the LAL-β-glucan reaction was not affected by the addition of polymyxin B, whereas the reaction between endotoxin and LAL was found to be inhibited by its addition. It is said that polymyxin B binds with the lipid A part of endotoxin and inhibits its activity. In addition, we found that even though the reaction between β-glucan and LAL was inhibited in the presence of an excess of carboxymethylated curdlan (CMC), the reaction between endotoxin and LAL was not affected by a similar addition 9).
Our endotoxin-specific LAL reagents (Limulus ES-Test Wako and pyroster™ ES-F) utilizes this principle of CMC excess. Ultimately, it is thought that β-glucan reacts with LAL via a pathway different from that of endotoxin.
The view that "LAL reacts to β-glucan" is not universally accepted. Compared to the data from those who believe LAL is not reactive to β-glucan, the data from those supporting reactivity, such as Iwanaga's group, is considered to be more detailed because it makes use of advanced technology. Of course, we are well-aware and believe that "LAL reacts to β-glucan".
Why don't you read through the literature and perform a replication study too, to find out?
References
- Kakinuma, A. et al. : Biochem. Biophys. Res. Commun., 101, 434 (1981)
- Morita, T. et al., FRBS Lett., 129, 318 (1981)
- Nakamura, T. et al. : Japanese Journal of Bacteriology, 38, 781 (1983)
- Pearson, F. C. et al. : Endotoxins and their detection with the Limulus amebocyte lysate test (Eds. : Watson, S. W. et al.), p 247 Alan R. Liss, Inc. : New York (1982)
- Söderhäll, K. et al. : Biol. Bull., 169, 661 (1985)
- Hodes, D. S. et al. : J. Clin. Microbiol., 25. 1701 (1987)
- Bayston, K. F. and Cohen, J. : J. Med. Microbiol., 31. 78 (1990)
- Tsuchiya, M. et al. : Chem, Pharm, Bull., 38. 2523 (1990)
- Tsuchiya, M. et al. : Japanese Journal of Bacteriology, 45, 903 (1990)




