BDNF Overview
This article was written by Dr. Nobuyuki Takei, Department of Brain Tumor Biology, Brain Research Institute, Niigata University.
Introduction
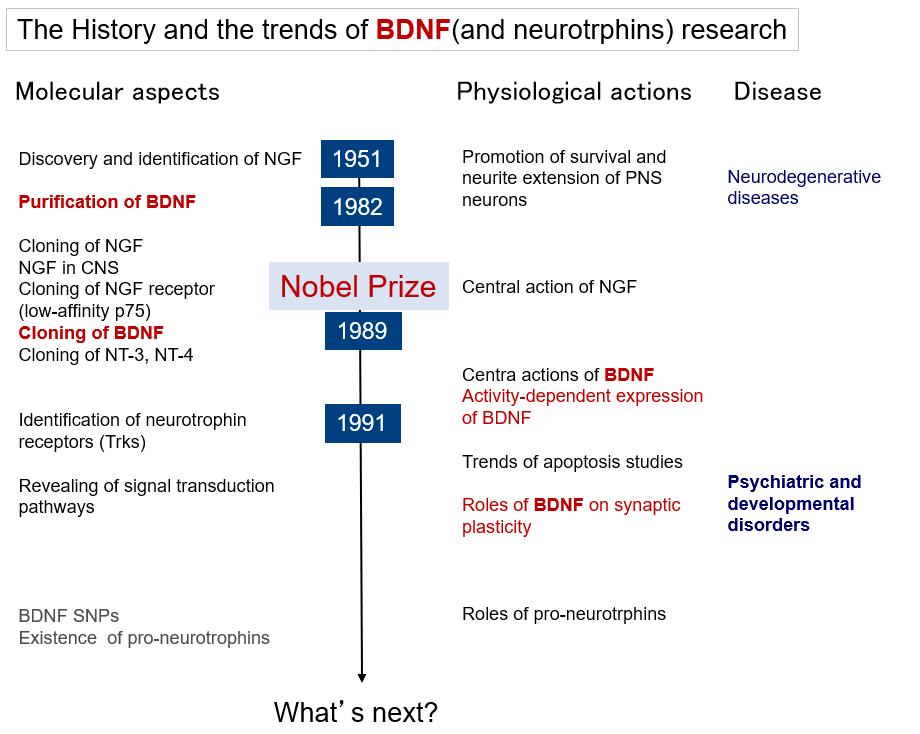
Research on brain-derived neurotrophic factor (BDNF) has evolved from its "classical" actions of promoting neuronal survival and differentiation to rather novel actions of regulating neurotransmission and synaptic plasticity. In line with this, interest in BDNF research in regards to brain diseases has shifted from its involvement in neurodegenerative diseases and therapeutic application for neuroprotection to its involvement in and treatment of psychiatric and developmental disorders that are thought to be caused by abnormalities in neural function (or abnormal circuit formation). Figure 1 illustrates the flow of BDNF research along the timeline, and this section provides a brief overview of BDNF.
BDNF and its receptor molecules
BDNF (Brain-Derived Neurotrophic Factor) is the most vital neurotrophic factor in the brain. Neurotrophic factor is a general term for proteins that maintain neuronal survival, induce differentiation (e.g., induction of neurite outgrowth and expression of neurotransmitter-synthesizing enzymes), promote maturation, and regulate synaptic functions. The discovery and identification of nerve growth factor (NGF) by Levi-Montalcini led to further research on neurotrophic factors. NGF affects only on specific neurons, suggesting the existence of other molecules with similar functions. After many attempts, BDNF was purified from porcine brain by Brade and Thoenen in 1982 (1). Subsequently, NGF was cloned in 1983 and BDNF in 1989 (2), and the two molecules exhibited more than 50% homology. This homologous region was used to clone a third and fourth factors, which were neurotrophin (NT)-3, and NT-4, respectively. These factors (NGF, BDNF, NT-3, and NT4) are called neurotrophin family. BDNF is composed of 112 amino acid residues, has a molecular weight of 13.5 KDa, an isoelectric point of 9.99, and, similar to NGF, has three intramolecular S-S bonds. Crystal structural analysis of NGF reveals that it has three flat β-sheets, through which it is thought to act physiologically as a homodimer. The domains that are not conserved among the family members are thought to define the specific binding ability to their cognate receptors (3).
NGF binding experiments suggest two types of receptors, low affinity and high affinity, with the low affinity receptor being identified as p75 (or p75NTR). This molecule binds to all neurotrophins with a low affinity (Kd=10-9 M), belongs to the tumor necrosis factor receptor family, is involved in apoptosis, and is again attracting attention as a receptor on which pro-neurotrophins act. In 1991, a high-affinity (Kd=10-11 M) receptor was identified. It was tropomyosin receptor kinase (Trk), which was originally identified as a gene product of the trk protooncogene found in cancer, but its ligand remains unknown. There are three types of Trks: TrkA, TrkB, and TrkC. TrkA (gene name: NTRK1) is a specific high-affinity receptor for NGF, TrkB (NTRK2) for BDNF and NT-4, and TrkC (NTRK3) for NT-3. All Trks consist of about 800 amino acids and undergo glycosylation to become a functional molecule with a molecular weight of 140-145 kDa. It is a receptor-type tyrosine kinase with an intracellular kinase domain. Upon binding to dimeric ligands, Trk itself also dimerizes and phosphorylates its tyrosine residues each other. The adaptor molecules bind to these phosphorylated tyrosines and transduce signals intracellularly, thereby exerting the biological activities of neurotrophins such as BDNF (4). The major intracellular signaling systems are common to receptor-type tyrosine kinases and include the 1) the PI3K-Akt system, which regulates apoptosis and translation, 2) the Ras-MAPK system, which induces differentiation mainly through transcriptional regulation, and 3) the PLCγ-Ca2+ system, which drives intracellular calcium signaling (5).
Physiological effects of BDNF
BDNF research initially focused on its effects on the peripheral nervous system. While NGF acts on a portion of the sympathetic neurons of dorsal root ganglia, BDNF acts on neurons of them that do not overlap with each other. In addition, BDNF shows the trophic effects on the sensory neurons of the nodose ganglia. The effects of neurotrophins on the peripheral nervous system are absolute and essential for survival and neurite extension. Therefore, each knockout mouse is lethal due to defects of peripheral nerves. Hence, the effects of neurotrophins on neuronal survival and neurite outgrowth are also referred to as "classical effects" of neurotrophic factors.
The focus of research then shifted to the central nervous system (CNS). Initially, NGF was found to acting on the basal forebrain cholinergic neurons, and BDNF acted on midbrain dopaminergic neurons and spinal motor neurons. These effects are also classical actions, and it has been reported that they induce the expression of s each neurotransmitter-synthesizing enzyme, increase the amounts of transmitters, and maintain survival. While the expression of NGF and TrkA is limited to a few neurons in the brain, BDNF and TrkB are abundantly found in the neurons in the brain. This has led to more extensive research on the effects of BDNF on CNS neurons, and it is now clear that BDNF is involved in synaptic plasticity dependent on neuronal activity, in addition to its classical effects of maintaining survival and inducing differentiation.
Neural plasticity is the reversible change in the efficiency of synaptic transmission through the regulation of neurotransmitter release and receptor functions, and the subsequent strengthening or elimination of specific neural circuits through synaptic reorganization. It does not necessarily require morphological changes, but can be caused by alterations in the quantity or variety of specific molecule, post-translational modifications (e.g., phosphorylation), or translocation of molecules, acting at the synapse through regulation of transcription, translation, and degradation. BDNF has been known to induce/enhance neurotransmitter release from CNS neurons and to enhance receptor functions, and it is now believed that it also acts to form/stabilize neural circuits in the long term. As a result, it is thought to be involved in higher brain functions such as learning and memory. (6,7).
Regulation of the expression and release of BDNF
BDNF expression is closely associated with neural activity. The stimulation of primary cultured neurons with glutamate considerably induced BDNF mRNA expression, whereas GABA stimulation decreased its expression (8). BDNF is expressed in neurons under physiological conditions, and it is upregulated by excitatory neurotransmission and downregulated by inhibitory neurotransmission (9). BDNF expression is also induced by long-term potentiation (LTP), which is considered the cellular basis of memory. BDNF mRNA expression levels have been observed in the visual cortex in response to sensory (visual) input. In addition, BDNF expression is upregulated in the brains of rats and monkeys after learning indicating activity-dependent induction of BDNF expression by activation of neural circuits in vivo (7).
Not only the regulation of expression, but also the release of BDNF is also neural activity-dependent. Considering the conventional classical action, neurotrophic factors, including but not limited to BDNF, are thought to be constitutively secreted. This is because a constant supply of a certain amount of BDNF is crucial for survival and other activities. However, a regulated release is necessary to elicit the rapid responses that are involved in synaptic transmission. In fact, BDNF is released in response to a depolarizing stimulus. Based on the mode of action of NGF in the peripheral nervous system, it was thought that neurotrophic factors are produced and secreted by the target cells to which axons are projected. However, BDNF in CNS neurons is contained within vesicles in the presynaptic region and is released in response to stimuli similar to neurotransmitters. This mechanism of expression and release also supports its involvement in neural plasticity, estabolishing that the mutual relationship between neural activity and BDNF (9).
BDNF and disease
The survival-promoting effects of neurotrophic factors have led to attempts at therapeutic intervention for neurodegenerative diseases. Although it is extreamly effective in in vitro and animal experiments, clinical trials have been unsuccessful (10). The primary issue is the molecular weight, which, being a protein, cannot generally cross the blood-brain barrier. Small molecule agonists have been developed and used in animal experiments, but their effects are still unclear. On the other hand, since BDNF is directly involved in neural functions, as discussed in this special issue, its role in psychiatric and developmental disorders, which are known as functional brain disorders, is attracting much attention. Although it's direct therapeutic use may be difficult for the reasons mentioned above, its role as a biomarker and the development of drugs that mimic the action of BDNF are expected to have great potential.
BDNF also has a central feeding suppression effect (11), and is apparently abundant in platelets, where it is released in a regulatory manner. Moreover, its association with obesity and lifestyle-related diseases such as diabetes and cardiovascular diseases has been reported, and its non-neuronal effects have also been clarified, leading to further expansion of research (12).
References
- Barde, Y. A. et al. : J., 1, 549 (1982).
- Leibrock, J. et al. : Nature, 14, 341149 (1989).
- Lewin, G. R. and Barde, Y. A. : Annu Rev. Neurosci., 19, 289 (1996).
- Deinhardt, K. and Chao, M. V. : Handb. Exp. Pharmacol., 220, 103 (2014).
- Huang, E. J. and Reichardt, L. F. : Annu Rev. Biochem., 72, 609 (2003).
- Thoenen, H. : Prog Brain. Res ., 128, 183(2000).
- Park, H. and Poo, M. M. : Nat Rev. Neurosci., 14(1), 7(2013).
- Lindholm, D. et al. : J. Neurobiol., 25, 1362(1994).
- Nawa, H. and Takei, N. : Trends Neurosci.,24 (12), 683 (2001).
- Thoenen, H. and Sendtner, M. : Nat. Neurosci.,1046 (2002).
- Takei, N. et al. : Front Psychol., 5, 1093 (2014).
- Marosi, K. and Mattson, M. P. : Tremds. Endocrinol. Metab., 25, 89 (2014).




