[Technical Report] Quantitation of Amino Acids in Beverages Containing Some Amino Acids Using Wakopak® Ultra APDS TAG®
This article was written by Yuki Suto, FUJIFILM Wako Pure Chemical Corporation, for Vol. 85, No. 4 (October 2017) of Wako Junyaku Jiho.
The content of this article is from the time of publication. It is not the latest information due to new knowledge and changes in regulatory rules after original publication.
Introduction
Since asparagine was discovered by isolating from the juice of asparagus in 1806, amino acids have been essential in our lives for the functions which are roughly classified into 4 categories: use as nutrients, use as taste components, use of its physiological actions, and use of its reactivity1). In addition, amino acids that cannot be synthesized by the animal body are essential amino acids for the animal, and important as nutrients in food.
In the market, there are wide range of foods containing amino acids such as beverages, drink preparations, and jelly, and thus, from now on also, many new products containing amino acids will be created.
Derivatization with 3-Aminopyridyl-N-Hydroxysuccinimidyl Carbamate (APDS)
The derivatization techniques for amino acid analysis in HPLC have long existed, and there are many derivatizing reagents including ninhydrin and o-phthalaldehyde. The amino acid derivatization method with APDS is an advantageous technique for reverse-phase LC/MS(/MS) because it increases the hydrophobicity and ionization efficiency of amino acids.2,3)
This method has been used in "AminoIndex® cancer screening (AICS)" of Ajinomoto Co., Inc.4), and also in the food analyses, there are analysis examples using UF-Amino Station, equipment dedicated to amino acid analysis.5)
Wakopak® Ultra APDS TAG® is a dedicated UHPLC column that enables the resolution and analysis of APDS-amino acid derivative using general-purpose LC/MS/MS equipment. We will introduce analyses of amino acids in commercial beverages using this column.
Experimental Method and Results
The flow of sample pretreatment and derivatization is shown in Figure 1, and the LC/MS/MS conditions are shown in Table 1. Quantification was performed by the internal standard method. The commercial beverage used in this study was labeled as containing 6 amino acids (glycine (Gly), alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), and arginine (Arg)). Amino acids from No. 13 to No. 20 in Table 1 are not contained in this commercial beverage.
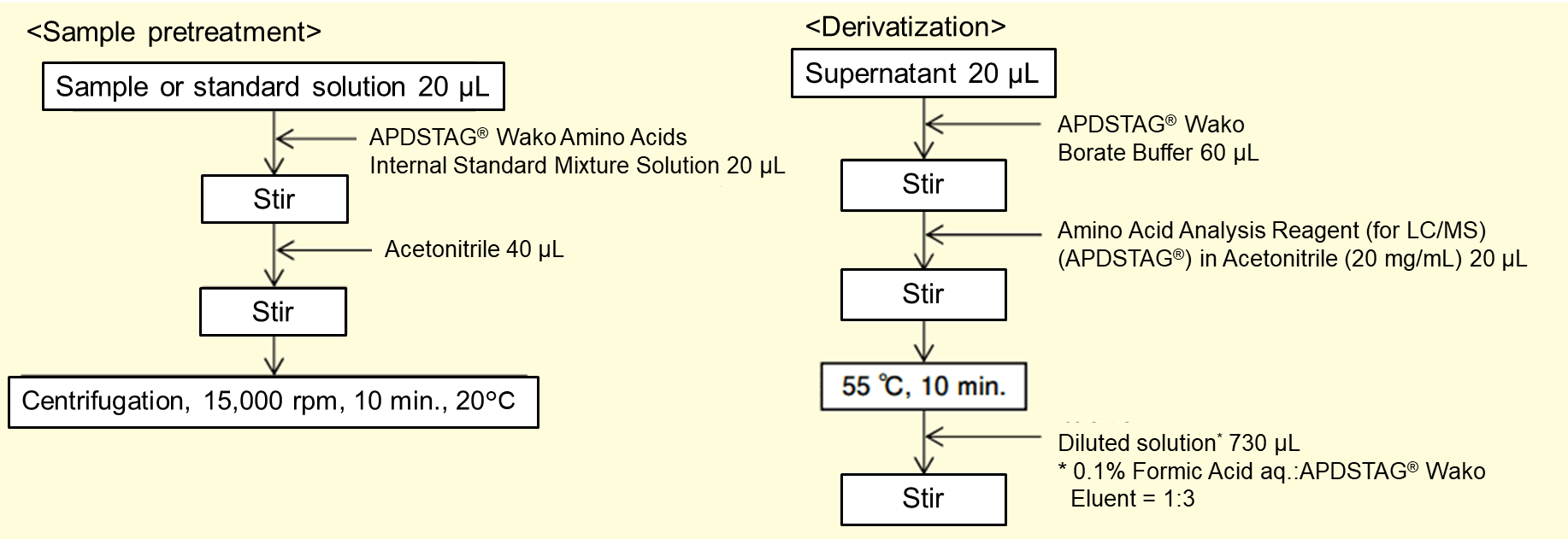 Figure 1. The flow of sample pretreatment and derivatization.
Figure 1. The flow of sample pretreatment and derivatization.
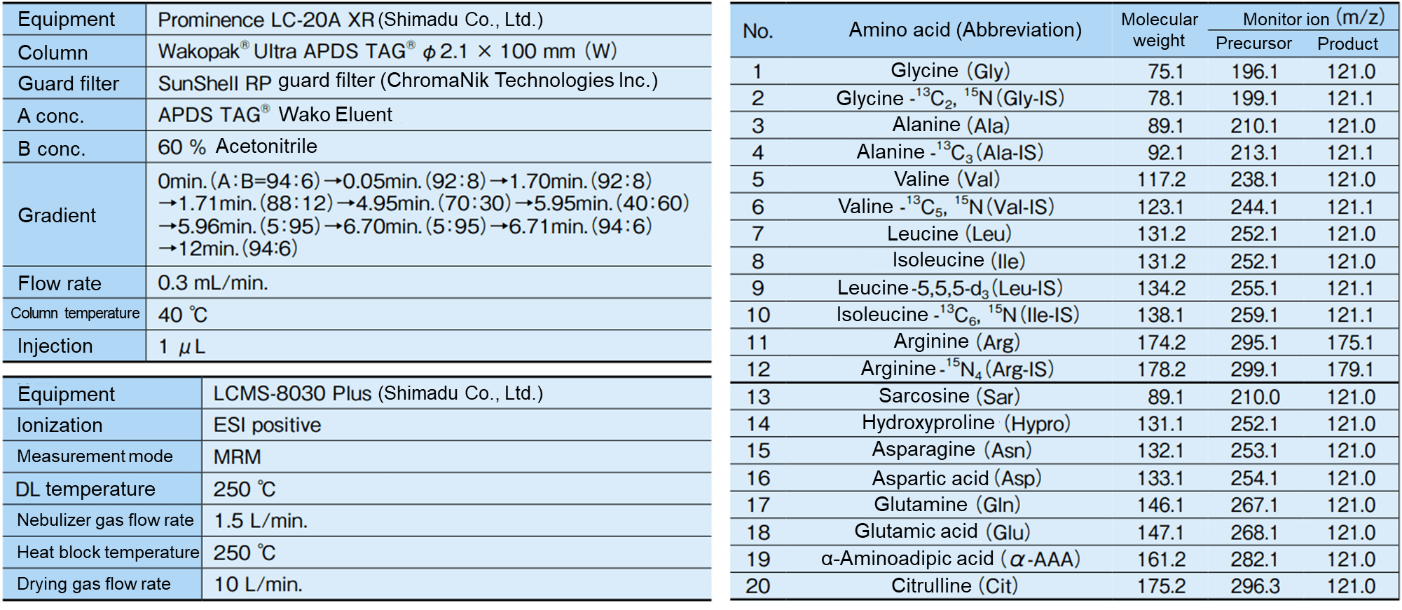 Table 1. LC/MS/MS conditions
Table 1. LC/MS/MS conditions
The MRM chromatogram of 6 amino acids in the commercial beverage is shown in Figure 2.
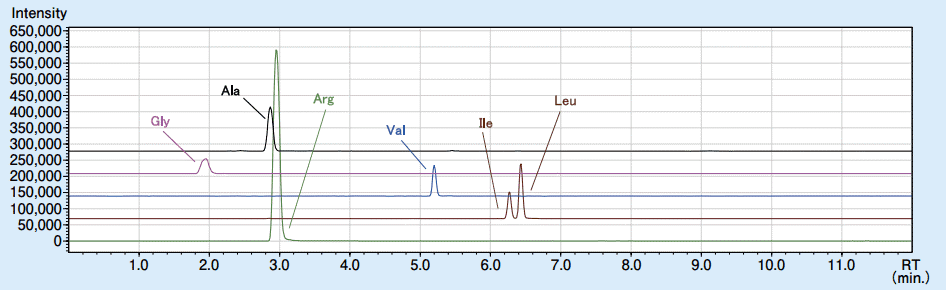 Figure 2. The MRM chromatogram of 6 amino acids in the commercial beverage
Figure 2. The MRM chromatogram of 6 amino acids in the commercial beverage
Next, the analytical standards of amino acids were spiked with the commercial beverage, and the spike and recovery test was performed. The MRM chromatogram of the commercial beverage after spiking the analytical standards is shown in Figure 3, and the results of the spiking and recovery test are shown in Figure 4. The recovery rate was 99-103%, showing a high recovery rate.
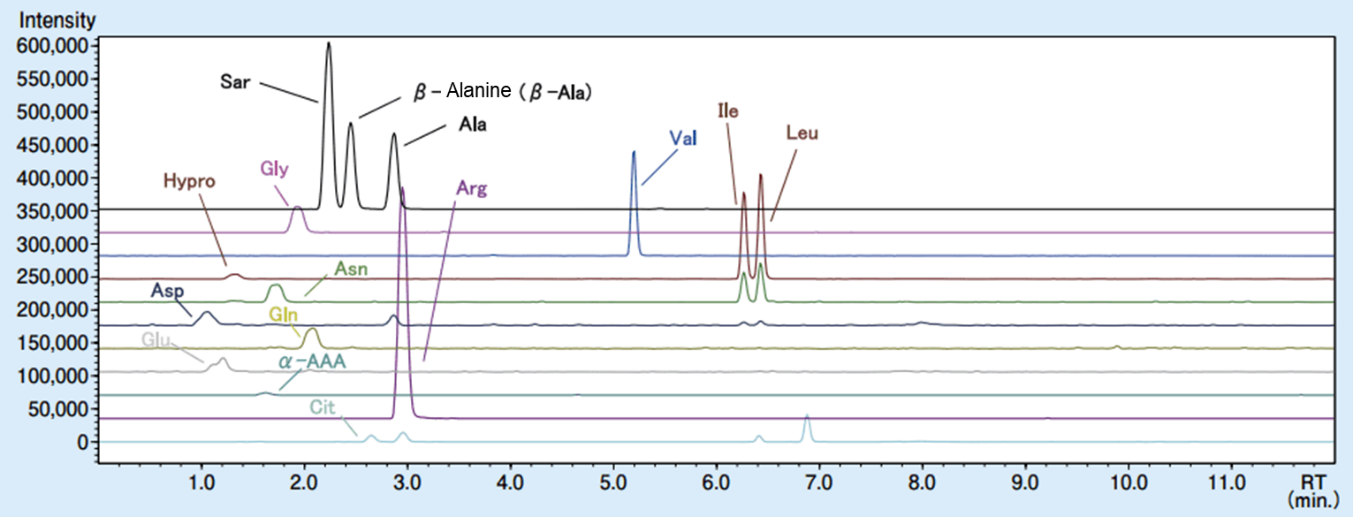 Figure 3. MRM chromatogram of commercial beverage after spiking analytical standards
Figure 3. MRM chromatogram of commercial beverage after spiking analytical standards
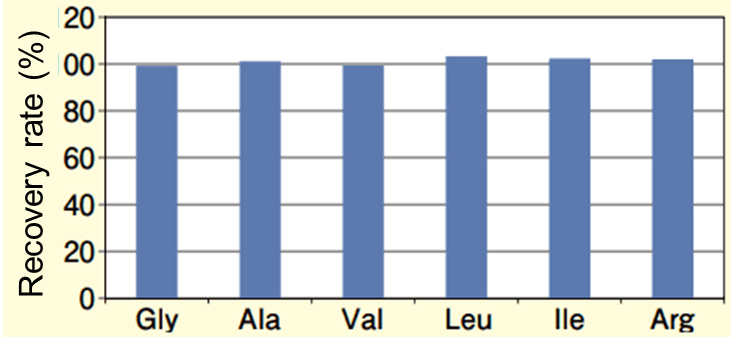 Figure 4. Results of spiking and recovery test
Figure 4. Results of spiking and recovery test
Based on the results of this study, the quantification of amino acids by APDS derivatization using Wakopak® Ultra APDS TAG® is considered effective. Although the results presented here are only for the beverage, it is considered useful also in the analysis of samples including other foods and culture fluids. Please utilize this UHPLC column for the quantification of amino acids.
Acknowledgements
We would like to express our deep appreciation to all members of Ajinomoto Co., Inc. for their cooperation in the study of Wakopak® Ultra APDS TAG®.
References
- Ajinomoto Co., Inc.: Amino Acid Handbook (Kogyo Chosakai Publishing Co., Ltd.) (2003).
- Patent No. 4453363.
- Miyano H.: Wako Junyaku Jiho, 79 (1), 2 (2011).
- Akira I.: KAGAKU TO SEIBUTSU, 53 (3), 192 (2015).
- Watanabe K. et al.: Shimadzu hyoron (Shimadzu review), 69 (1.2), 47 (2012).




