[Technical Report] Analysis examples of proficiency substances by "ADRA", a method for the evaluation of skin sensitization, using Wakopak® Core C18 ADRA
This article was written by Yuki Suto, FUJIFILM Wako Pure Chemical Corporation, for Vol. 87, No.4 (October 2019) of Wako Junyaku Jiho.
The content of this article is from the time of publication. It is not the latest information due to new knowledge and changes in regulatory rules after original publication.
Introduction
In recent years, establishment of regulations for animal testing have been encouraged from the viewpoints of 3Rs principles (replacement, reduction, and refinement) for animal testing and welfare. Especially, in EU, animal testing for cosmetics has been prohibited stepwise since 1993, and the EU directive1 intended to enforce the total ban on marketing of cosmetics tested on animals after 2013 was issued in 2003. The relevant regulations were established2 in 2009.
In such situations, development of alternatives to animal experiments has been required worldwide. In Japan, the Japanese Center for the Validation of Alternative Methods, National Institute of Health Science (JaCVAM) was founded in 2005. Since then, many alternative testing methods developed in Japan have been listed in the test guidelines issued by the Organisation for Economic Co-operation and Development (OECD) (OECD Test Guidelines).3
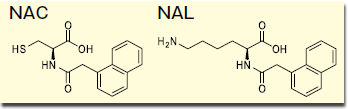
Figure 1. Structural formula of NAC and NAL
Wakopak® Core C18 ADRA is a column that was developed by FUJIFILM Corporation and conforms to requirements for an alternative testing for skin sensitization, Amino acid Derivative Reactivity Assay (ADRA),4 listed in the OECD Test Guidelines on June 18, 2019. In ADRA, each test chemical is mixed with N-(-2(-1-Naphthyl)acetyl)-L-cysteine (NAC) and α-N-(-2(-1-Naphthyl)acetyl-L-lysine (NAL) (Figure 1), and the decreased rates of NAC and NAL are monitored as indices of the reactivity of the test chemical. This document shows examples of analyses on 10 proficiency substances, which are required to be successfully performed for technical qualification for ADRA operations.
For details of ADRA and differences from the existing technique of Direct Peptide Reactivity Assay (DPRA), see the references.5-9
Testing method
In this document, the testing was performed with the ADRA kit (code No. 296-80901) in accordance with the attached protocol. In this testing, however, polypropylene microtubes were used for the reaction instead of a 96-well plate indicated in the original ADRA. Table 1 shows liquid chromatography (LC) conditions. It should be noted that the equilibration time after the retention time of 13.5 minutes in each run may differ because it depends on configuration of instruments and mixer volume.
Table 1. LC conditions
| Instrument | Agilent UHPLC 1290 infinity Ⅱ (Agilent Corporation) |
|---|---|
| Column | Wakopak® Core C18 ADRA 3.0 x 150 mm (D) |
| Mobile phase A | 0.1% Trifluoroacetic acid in water |
| Mobile phase B | 0.1% Trifluoroacetic acid in acetonitrile |
| Gradient condition for NAC | 0 min. (A:B= 70: 30)→ 9.5 min. (45: 55)→ 10.0 min (0: 100)→ 13.0 min. (0: 100)→ 13.5 min. (70: 30)→ 25.0 min. (70: 30) |
| Gradient condition for NAL | 0 min. (A:B= 80: 20)→ 9.5 min. (55: 45)→ 10.0 min (0: 100)→ 13.0 min. (0: 100)→ 13.5 min. (80: 20)→ 25.0 min. (80: 20) |
| Flow rate | 0.3 mL/min. |
| Mixer volume | 35 µL |
| Column temperature | 40 ℃ |
| Wavelength | UV 281 nm |
| Injection volume | 10 µL |
Results
Table 2 shows evaluation results on proficiency substances, and Figure 2 shows representative chromatograms. The decreased rates for the proficiency substances obtained with the Wakopak® Core C18 ADRA fell within the ranges presented in the guideline for ADRA.
FUJIFILM Wako's website offers videos for testing operations with the kit and the protocols. We are always pleased to help your ADRA operations with our products and information.
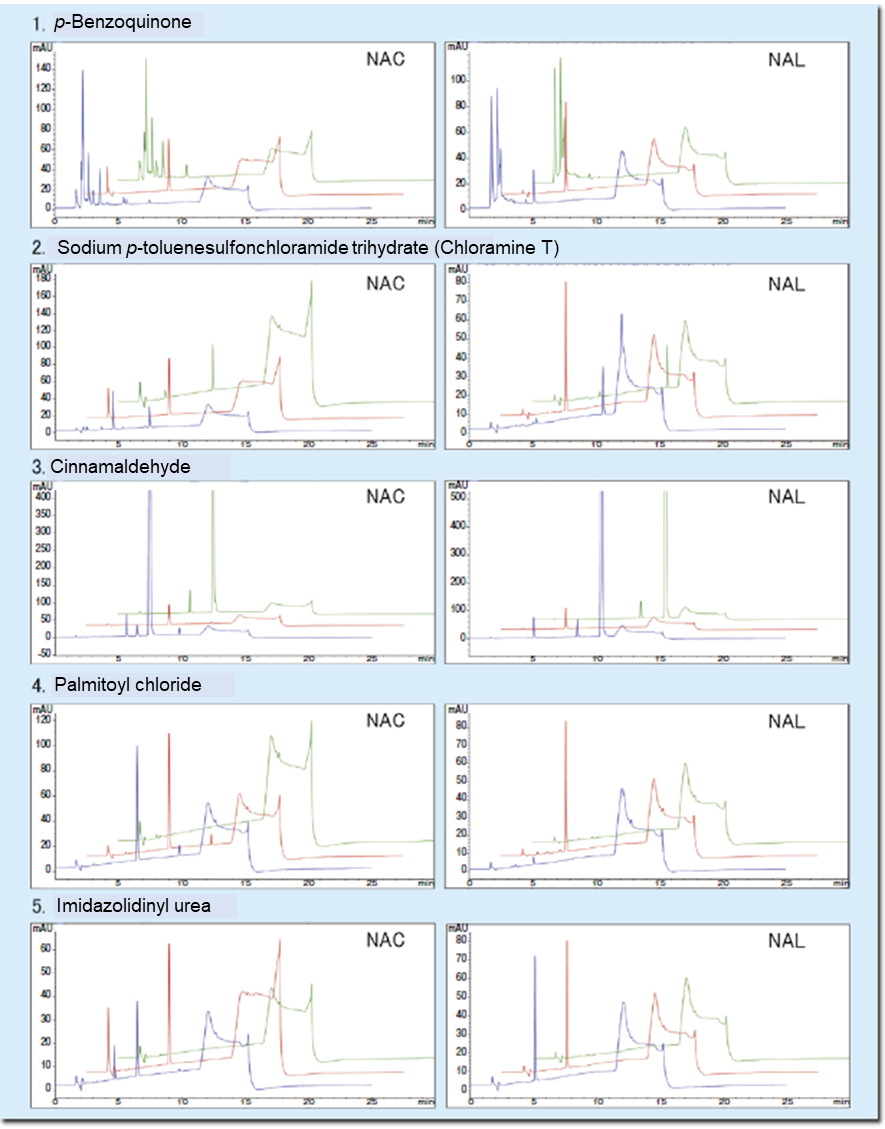
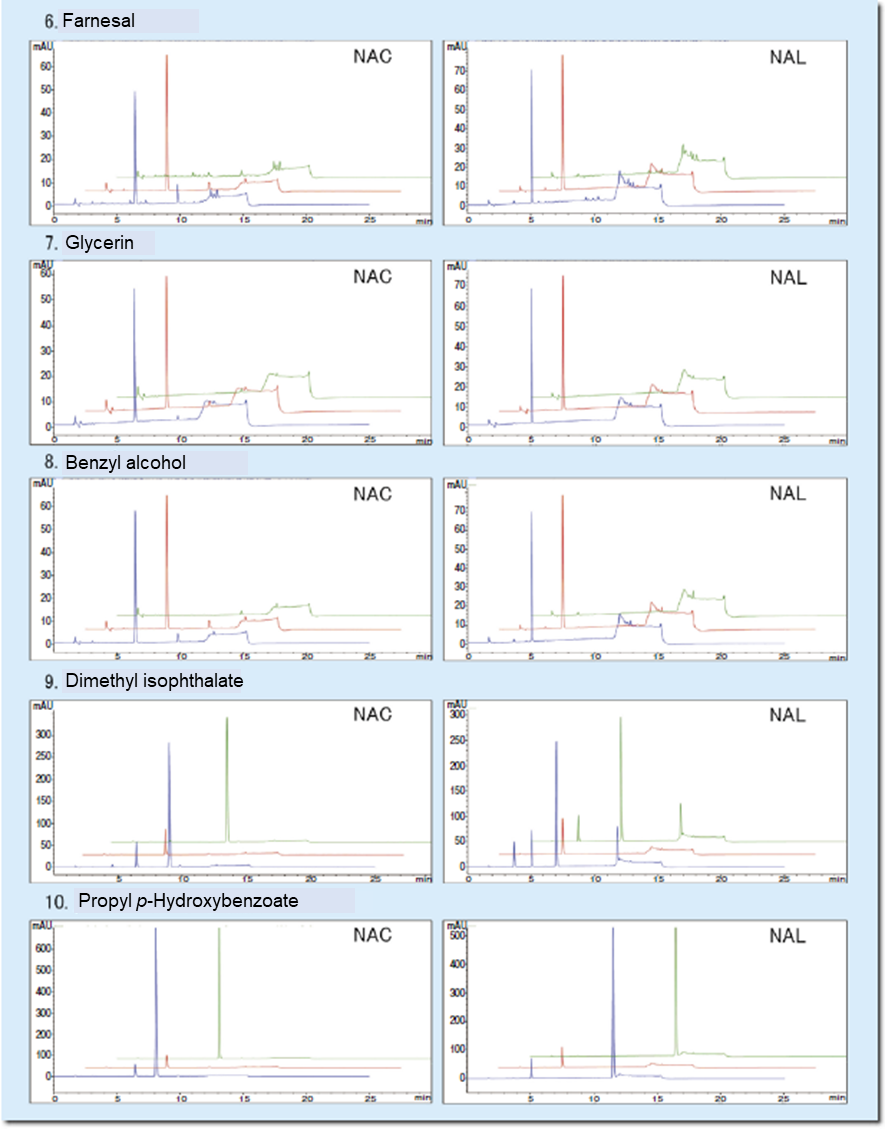
Figure 2. Chromatograms of proficiency substances
Green: Test chemical
Red: NAC or NAL
Blue: Test chemical + NAC or NAL
Table 2. List of proficiency substances and evaluation results
| No. | Test chemical | CAS RN® | Description | Molecular weight | Solvent | Prediction by ADRA | Known decreased rate (%) | Measured decreased rate (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| NAC | NAL | NAC | NAL | |||||||
| 1 | p-Benzoquinone | 106-51-4 | Solid | 108.09 | Water | Positive | 90-100 | 40-70 | 100 | 61 |
| 2 | Sodium p-toluenesulfonchloramide trihydrate (Chloramine T) |
7080-50-4 | Solid | 281.69 | Water | Positive | 90-100 | 90-100 | 100 | 97 |
| 3 | Cinnamaldehyde | 14371-10-9 | Liquid | 132.16 | Acetonitrile | Positive | 40-100 | ≦ 20 | 43 | 1 |
| 4 | Palmitoyl chloride | 112-67-4 | Liquid | 274.87 | Acetonitrile | Positive | ≦ 10 | 50-100 | 2 | 93 |
| 5 | Imidazolidinyl urea | 39236-46-9 | Solid | 388.29 | Water | Positive | 10-45 | ≦ 10 | 35 | 1 |
| 6 | Farnesal | 19317-11-4 | Liquid | 220.35 | Acetonitrile | Positive | 10-40* | ≦ 15 | 15 | 2 |
| 7 | Glycerin | 56-81-5 | Liquid | 92.09 | Water | Negative | ≦ 7 | ≦ 7 | 0 | 0 |
| 8 | Benzyl alcohol | 100-51-6 | Liquid | 108.14 | Acetonitrile | Negative | ≦ 7 | ≦ 7 | 0 | 0 |
| 9 | Dimethyl isophthalate | 1459-93-4 | Solid | 194.19 | Acetonitrile | Negative | ≦ 7 | ≦ 7 | 0 | 0 |
| 10 | Propyl p-Hydroxybenzoate | 94-13-3 | Solid | 180.20 | Acetonitrile | Negative | ≦ 7 | ≦ 7 | 0 | 0 |
* The range will be revised, although it is different from the guideline.
References
- European Commission : "DIRECTIVE 2003/15/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 27 February 2003, amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products, Official J. European Union, L66/26(2003).
- European Commission : "REGULATION(EC) No 1223/2009 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 30 November 2009 oncosmetic products", Official J. European Union, L 342/59(2009).
- Miyazaki, H. and Yoshiyama, Y.: Folia Pharmacologica Japonica, 151,48(2018).
- OECD:"In Chemico Skin Sensitisation : Amino acid Derivative Reactivity Assay(ADRA)", OECD Guideline for the Testing of Chemicals 442C(2019).
- Yamamoto, Y. et al.: Wako Junyaku Jiho 87(1), 12(2019).
- Fujita, M. et al. : J. Pharmacol. Toxicol. Methods, 70, 94(2014).
- Yamamoto, Y. et al. : J. Appl. Toxicol., 35, 1348(2015).
- Fujita, M. et al. : J. Appl. Toxicol., 39, 191(2019).
- Fujita, M. et al. : Toxicol. in Vitro, 59, 161(2019).




