What is Endotoxin?
This article was written by Dr. Masakazu Tsuchiya, FUJIFILM Wako Pure Chemical Corporation,
for No. 2 (October 1990) of Wako Junyaku Jiho.
The content of this article is from the time of publication. It is not the latest information due to new
knowledge and changes in regulatory rules after original publication.
What is ENDOTOXIN?
Endotoxin (also known as lipopolysaccharide or LPS) is a type of pyrogen which possesses various biological activities, and is found in the outer membrane of Gram-negative bacteria. Due to its strong pyrogenicity, it has been feared that endotoxin could contaminate injectable drugs. It has been mainly detected through use of the rabbit pyrogen test, but the limulus test has been designed to detect bacterial endotoxin in vitro. The structure and biological activities of endotoxin have been well-researched1, 2), thus in this article we will discuss the complications of endotoxin with regard to the limulus test.
Heat Stability of Endotoxin
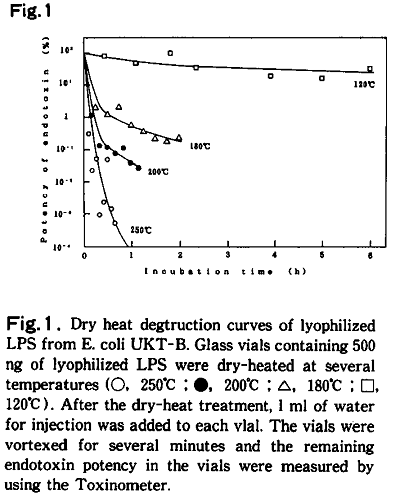
Apparatus used in the limulus test are generally made endotoxin-free with dry heat sterilization. Now, to what extent do items have to be dry heat sterilized to inactivate endotoxin? Endotoxin has long been known as a heat-stable toxin, and it is said that a harsh condition of 250℃ for more than 30 minutes is necessary to sufficiently inactivate endotoxin with dry heat sterilization. In our experiment, which used 500ng of LPS derived from E. coli UKT-B strain, endotoxins were not sufficiently inactivated with heat under normal sterilization conditions, that is 180℃ or 200℃, and they only reached below detection limit under the condition of 250℃ for 1 hour (Fig.1)3).
Thus, it seems that apparatus used in the limulus test need to be dry heat sterilized with the appropriate temperature and time after thorough washing to reduce contamination as much as possible; our standard is 250℃ for 2 hours.
Endotoxin is not always heat stable in all circumstances; we have observed that endotoxin activity in a low-concentration aqueous solution can be reduced by heating. In our experiment, the activity of a low- concentration endotoxin solution was reduced at 70℃, even though the activity was stable at 37℃ for at least 24 hours (Fig.2)3).
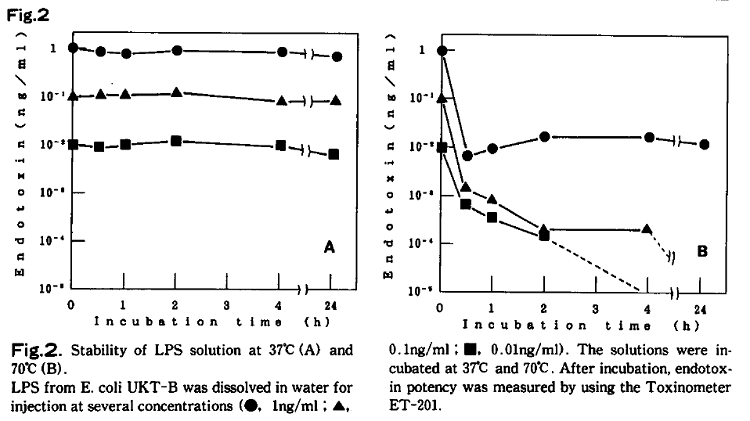
It is also common to fail to detect the activity of a very small amount of endotoxin in a solution after autoclaving. In other words, endotoxin aqueous solutions are not as stable to heat as dry endotoxin, and at a low concentration it is possible to reduce endotoxin activity to below detection limit with heating. However, confirmation testing through the limulus test must be performed when inactivating endotoxin with methods other than dry heat sterilization.
Our next post will be a continuation of Episode 2 "Endotoxin," and will discuss "Reference Standard Endotoxin and Control Standard Endotoxin."
Reference Standard Endotoxin and Control Standard Endotoxin
The United States Pharmacopeia defines the national endotoxin reference standard as Reference Standard Endotoxin (RSE). Other endotoxin standards that have been standardized against RSE are defined as Control Standard Endotoxin (CSE) 4). The potency of CSE needs to be determined based on RSE and this potency determination must be performed every time the LAL lot or CSE lot changes. This FDA regulation implies that the conversion factor of CSE might change between different CSE lots and also between different LAL lots. This factor must be considered when performing the limulus test.
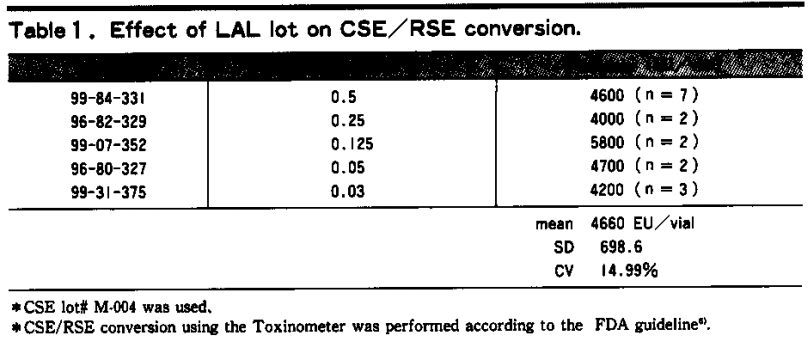
When determining the potency of the CSE we prepared against the US RSE shown in Table 1, the relative potency tended to change when the LAL lot changed 5). This confirms that when using CSE it is necessary to determine the relative potency every time the LAL reagent lot changes. Therefore, to reduce the number of CSE potency determination tests, it is more economical to use lots of LAL that are as large as possible.
The current US RSE is a lyophilized product prepared from Lipopolysaccharide (LPS) derived from E. coli strain O113:H10 and has lactose and polyethylene glycol 6000 as additives 6). This reference standard has been adopted as the Office of Biologics (OB) US Standard Endotoxin (Lot EC-5) and the US Pharmacopeia (USP) Endotoxin Reference Standard (Lot F).
The Japanese Pharmacopeia uses a national endotoxin reference standard prepared from LPS derived from E. coli strain UKT-B and uses mannitol as an additive 7). This reference standard endotoxin was determined to have a potency of 8 EU/ng based on a comparison with US RSE through the rabbit pyrogen test and the limulus Test 8). This value was obtained statistically from a large quantity of data and may change slightly depending on the LAL lot. The Japanese Pharmacopeia does not mention the use of CSE and for the time being it seems necessary to use the national reference standard in final tests.
As explained above, the relative potency of an endotoxin standard may change depending on the LAL lot it is tested with. It is therefore possible that measured endotoxin concentration in a sample will change depending on the LAL lot, even if the sample is measured alongside the same standard as a control. This variance is due to reasons such as unknown sources of endotoxins in samples, and given the diversity of endotoxin structures this phenomenon may not be surprising after all.
Of course, as shown in Table 1, the potency variance may not be enormous and for the time being it is recommended to measure samples against the appropriate standard. It is particularly important to use the RSE associated with each country in which drug safety is tested using the limulus test.
References
- Honma, Y. (Ed.): Endotoxins: their structures and activities, Ishiyaku Publishers, Inc. Tokyo (1983)
- Handbook of Endotoxin (Ed, : Proctor, R.A.), vol.1-4, Elsevier, Amsterdam (1986)
- Tsuchiya, M. : Journal of Antibacterial and Antifungal Agents, Japan, 18, 287 (1990)
- The United States Pharmacopeia 22th, p. 1493, Pharmacopeial Convention Inc., MD (1989).
- Tsuchiya, M.: The Society for Antibacterial and Antifungal Agents, Japan, 18, 287 (1990)
- Hochstein, H.D. et al : J. Biol. Standardization, 11.251 (1983)
- Supplement B-11 to the Japanese Pharmacopeia 11th Edition (1988)
- Research Report for Public-Private Joint Project of Longevity-related Basic Science Research Project, 2nd field, p.184, Human Science Promotion Foundation, Tokyo (1998)
- Guideline on validation of the limulus amebocyte lysate test as an end-product endotoxin test for human and animal parenteral drugs, biological products, and medical devices (1987) FDA.




