Oxytocin ELISA Kit Wako
- for Immunochemistry
- Manufacturer :
- FUJIFILM Wako Pure Chemical Corporation
- Storage Condition :
- Keep at 2-10 degrees C.
- GHS :
-
- Label
- SDS
|
Comparison
|
Product Number
|
Package Size
|
Price
|
Availability
|
Certificate of Analysis
|
Purchase |
|---|---|---|---|---|---|---|
|
|
|
96Tests
|
|
Out of Stock |
※Check availability in the US with the distributor.
Document
Kit component
96 tests
| Antibody-coated Plate | 1 plate |
|---|---|
| Oxytocin Standard | 100 µL |
| Buffer | 60 mL |
| Biotin-conjugated Oxytocin | 1 vial |
| Peroxidase-conjugated Streptavidin Solution | 100 µL |
| Luminescent Reagent 1 | 6 mL |
| Luminescent Reagent 2 | 6 mL |
| Wash Solution (10x) | 100 mL |
| Sample Buffer 1 | 5 mL |
| Sample Buffer 2 | 12 mL |
| Plate Seal | 4 sheets |
Product Overview
Oxytocin is often called the "happy hormone" or "love hormone", as it has stress-relieving, anti-anxiety, and fear-alleviating effects and plays a role in the development of maternal behavior. It is a molecule that has attracted attention in treating mental disorders such as depression and autism or developing functional food materials. However, the assay of oxytocin used to include the need for complicated pretreatment using a C18 column, and a large amount of sample.
Oxytocin ELISA Kit Wako is an ELISA kit that can determine the amount of oxytocin in a sample. It overcomes issues with conventional oxytocin assays, since sample pretreatment simply requires mixing, stirring, and centrifuging of reagents, and the minimum sample amount required is 50 μL.
Information
This kit is an upgraded kit and replaces 292-84401 Oxytocin ELISA Kit Wako. It has been confirmed that there is a correlation between the measured values of this product and those of conventional products. (Please see "Correlation with conventional product")
Features
- Easy-to-use pretreatment
Sample pretreatment requires simply adding pretreatment solutions, stirring, and centrifuging. A C18 column and organic solvent are not required. - Can be assayed with a tiny sample volume
Minimum sample amount required is 50 µL (n=1). - Short assay time
Assay time is approximately 2.5 hours. Pretreatment can be done in approximately 30 minutes. - Can be used with a variety of samples
Human saliva/urine/serum/plasma
mouse or rat serum/plasma
[Note] A plate reader for luminescence measurement is required
Kit Performance
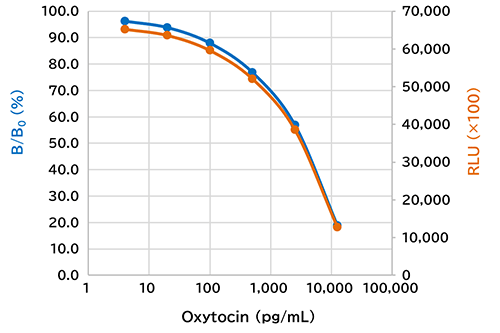
| Calibration curve range | 4.00-12,500 pg/mL |
|---|---|
| Assay target | Oxytocin |
| Analysis sample | Human saliva/urine/serum/plasma Mouse serum/plasma Rat serum/plasma |
| Sample volume | 50 μL (Required amount for n=1) 200 μL (Recommended amount for n=2) |
| Measurement duration | Approximately 2.5 hours |
| Detection method | Luminescence (a plate reader for luminescence detection is required) |
Example of Calibration Curve
Assay Principles
This kit is based on a competitive assay, and luminescence intensity decreases as the amount of oxytocin in the sample increases.
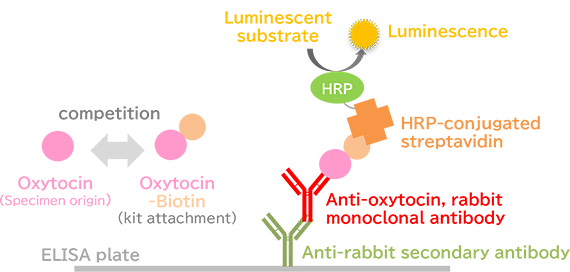
- In advance, anti-oxytocin, rabbit monoclonal antibody, and anti-rabbit secondary antibody are solid phased in each well of the assay plate.
- Add oxytocin-biotin and the standard solution or the pretreated sample to wells of the assay plate. Allow them to react competitively.
- Allow biotin and HRP-conjugated streptavidin to react.
- Add a luminescent substrate for HRP.
- Assay the oxytocin concentration in the sample by measuring HRP activity (luminescence intensity) in the wells.
Sample Pretreatment Methods
Pretreatment method of FUJIFILM Wako
(Approx. 30 minutes)
Sample (saliva/urine/serum/plasma)
↓
Add Sample Buffer 1
↓
After stirring, allow to stand at room temperature for 10 minutes
(stir once after 5 minutes have elapsed)
↓
Add Sample Buffer 2
↓
After stirring, allow to stand at room temperature for 10 minutes
(stir once after 5 minutes have elapsed)
↓
Centrifuge
↓
Samples to be measured consist of separated supernatant
Pretreatment method of competitive company E
(Approx. 2-4 hours)
Sample (saliva/urine/serum/plasma)
↓
Add trifluoroacetic acid (TFA), and stir
↓
Centrifuge
↓
Add separated supernatant to a C18 column that was equilibrated with TFA and acetonitrile
↓
Wash with TFA and elute with acetonitrile
↓
Decompress and dry the elution fraction
↓
Dissolve with buffer to make samples to be measured
Protocol
The video provides the protocol and tips for experiments using the Oxytocin ELISA Kit Wako.
【Protocol】Oxytocin ELISA Kit Wako(Youtube 4:57)
Data
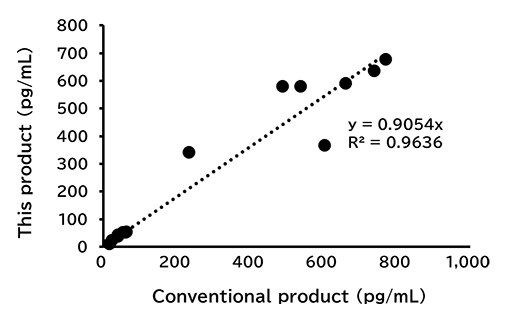
Correlation with conventional product
For human saliva/serum/plasma(EDTA), mouse serum/plasma(EDTA) and rat serum/plasma(EDTA) samples, measurements were conducted using this kit and conventional product (Product No. 292-84401). The measurement values obtained using both products were then compared.
(1) Total
(2) Low concentration range
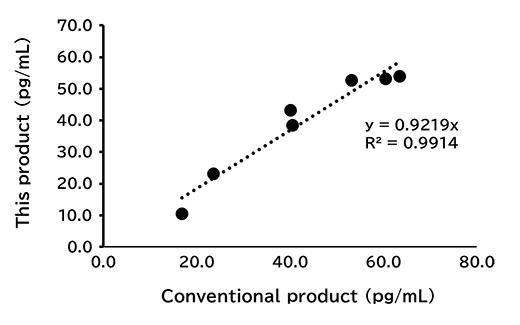
[Result]
A good correlation was confirmed.
Spiked recovery test
Human
| Amount spiked (pg/mL) |
Measured value (pg/mL) |
Recovery volume (pg/mL) |
Recovery rate (%) |
|
|---|---|---|---|---|
| Saliva | 0 | 4.10 | - | - |
| 50 | 49.5 | 45.4 | 90.8 | |
| 500 | 530 | 526 | 105 | |
| 1,000 | 930 | 926 | 92.6 | |
| Average | 96.1 | |||
| Urine | 0 | 28.4 | - | - |
| 50 | 80.2 | 51.8 | 104 | |
| 500 | 512 | 484 | 96.8 | |
| 1,000 | 952 | 924 | 92.4 | |
| Average | 97.7 | |||
| Serum | 0 | 47.2 | - | - |
| 50 | 97.9 | 50.7 | 101 | |
| 500 | 522 | 475 | 95.0 | |
| 1,000 | 1,108 | 1,061 | 106 | |
| Average | 101 | |||
| Plasma (EDTA) |
0 | 30.5 | - | - |
| 50 | 74.7 | 44.2 | 88.4 | |
| 500 | 497 | 467 | 93.4 | |
| 1,000 | 962 | 932 | 93.2 | |
| Average | 91.7 | |||
| Plasma (Heparin) |
0 | 8.50 | - | - |
| 50 | 57.0 | 48.5 | 97.0 | |
| 500 | 509 | 501 | 100 | |
| 1,000 | 983 | 975 | 97.5 | |
| Average | 98.2 | |||
| Plasma (Citrate) |
0 | 15.6 | - | - |
| 50 | 69.0 | 53.4 | 107 | |
| 500 | 465 | 449 | 89.8 | |
| 1,000 | 927 | 911 | 91.1 | |
| Average | 96.0 | |||
Mouse
| Amount spiked (pg/mL) |
Measured value (pg/mL) |
Recovery volume (pg/mL) |
Recovery rate (%) |
|
|---|---|---|---|---|
| Serum | 0 | 16.4 | - | - |
| 50 | 67.2 | 50.8 | 102 | |
| 500 | 582 | 566 | 113 | |
| 1,000 | 995 | 979 | 97.9 | |
| Average | 104 | |||
| Plasma (EDTA) |
0 | 5.34 | - | - |
| 50 | 59.8 | 54.5 | 109 | |
| 500 | 476 | 471 | 94.2 | |
| 1,000 | 988 | 983 | 98.3 | |
| Average | 101 | |||
Rat
| Amount spiked (pg/mL) |
Measured value (pg/mL) |
Recovery volume (pg/mL) |
Recovery rate (%) |
|
|---|---|---|---|---|
| Serum | 0 | 3.70* | - | - |
| 50 | 50.4 | 46.7 | 93.4 | |
| 500 | 545 | 541 | 108 | |
| 1,000 | 911 | 907 | 90.7 | |
| Average | 97.4 | |||
| Plasma (EDTA) |
0 | 6.50 | - | - |
| 50 | 56.1 | 49.6 | 99.2 | |
| 500 | 501 | 495 | 99.0 | |
| 1,000 | 888 | 882 | 88.2 | |
| Average | 95.5 | |||
* Reference value
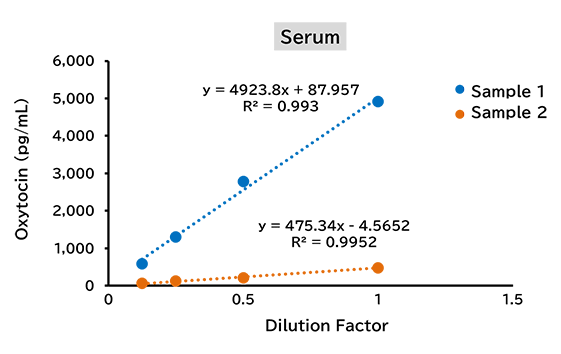
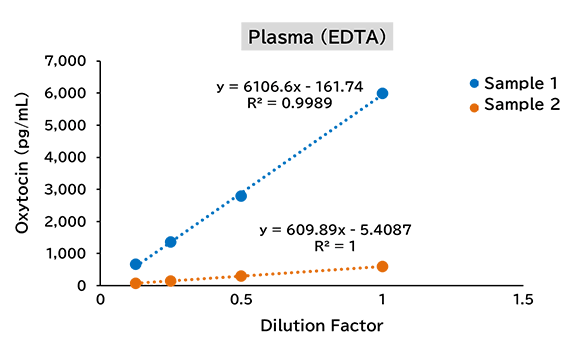
Dilution linearity test
(1) Human
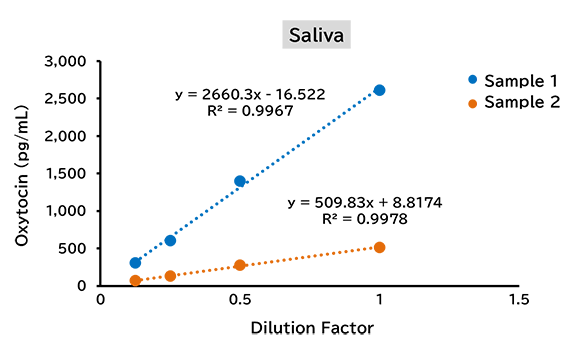
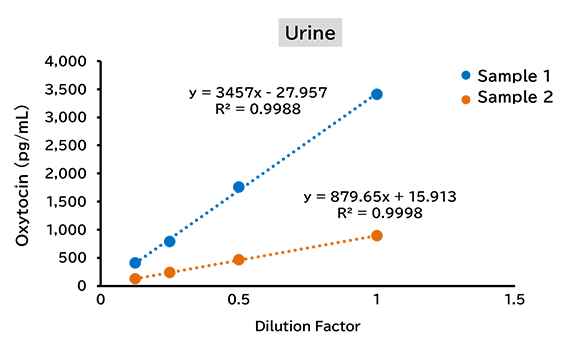
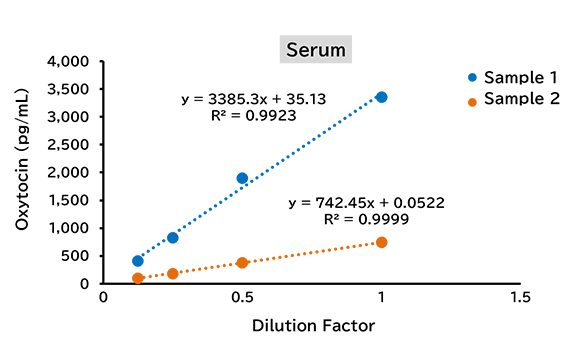
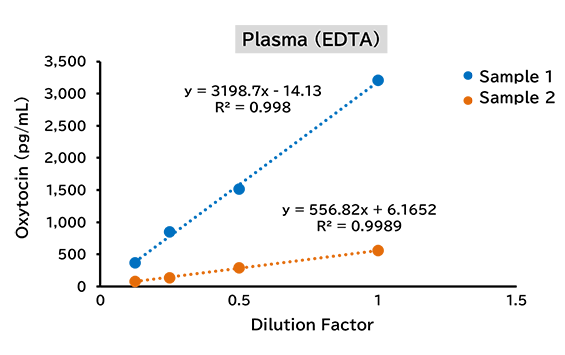
(2) Mouse
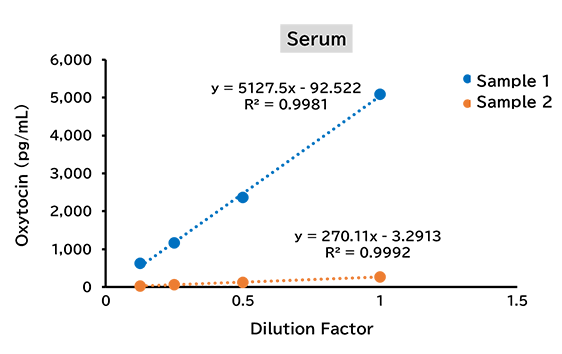
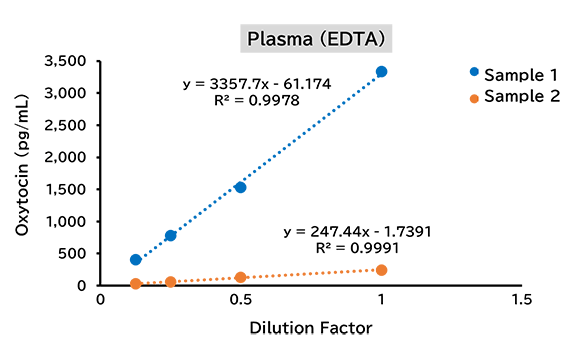
(3) Rat
References
Citations for this product
- Nakata, M. et al.: J. Mol. Sci., 24(9), 8248(2023). [Sample: Mouse plasma]
1, 5-Anhydro-D-Fructose Exhibits Satiety Effects via the Activation of Oxytocin Neurons in the Paraventricular Nucleus - Shima,T. et al.: Behav., 283, 114623(2024). [Sample: Human saliva]
Augmented-reality-based multi-person exercise has more beneficial effects on mood state and oxytocin secretion than standard solitary exercise
[Note] 1 - 2) use the predecessor of this kit, Oxytocin ELISA Kit Wako (Product Number 292-84401, discontinued) is used.
FAQ
About sample
- What types of samples can be used?
- The assay can be performed with human saliva/serum/plasma/urine, mouse serum/plasma, and rat serum/plasma.
- What is the required sample volume?
- The minimum sample volume required is 50 µL (n=1). The measurement can also be performed by diluting with the buffer solution provided in the kit. A sample volume of 200 µL is recommended for duplicate measurements. Thevolume of 200 µL is necessary to separate the supernatant without disturbing the “gel-containing pellet” generated after addingPretreatment Solution 2 and centrifugation. (In theory, if the gel is not disturbed, sample volumes less than 200 µL can be used.)
- How should samples be stored?
- For long-term storage of samples, add the protease inhibitor aprotinin at 100 KIU/mL (example: Product Number 018-18111) and the preservative Proclin™ 950 at 1/750 volume to the samples, and store at -80°C or lower.
For saliva samples, assay results did not change significantly after storage at room temperature for 6 hours, in a refrigerator for 24hours at -20°C for 1 month or at -80°C for 6 months without adding aprotinin and Proclin™ 950. (Fluctuations in room temperature were within ±15% of the temperature at hour 0.) Repeated freezing and thawing should be avoided whenever possible. Fujifilm Wako has observed that assay results fluctuated 15% or more after four or more cycles of freezing and thawing.
- What is the recommended method of collection of saliva samples?
- The passive drool method is recommended for the collection of saliva samples. (the passive drool method requires subjects to salivate into a vial or tube.)
It has been confirmed that samples collected using cotton swabs or inert polymer swabs can be used similarly to samples collected by the passive drool method. However, caution should be taken because some swab materials may absorb target analytes.
- Which anticoagulants can I use when using plasma as sample?
- Anticoagulants such as heparin, citrate and EDTA do not significantly influence the assay when used in normal amounts.
About pretreatment
- Is it possible to extend the standing time during sample pretreatment?
- Do not extend the processing time for Sample Pretreatment Solution 1. Since Pretreatment Solution 1 contains acid, the sample becomes acidic on its addition. A prolonged standing period may cause denaturation of the sample matrix. A slight extension (approximately 1 to 4 minutes) of the processing time for Sample Pretreatment Solution 2 is acceptable. The processing time must not be shorter than the standard time of 5 minutes.
- Is there any point to pay special attention to during pretreatment?
- Since the gel contained in Sample Pretreatment Solution 2 settles over time after stirring, stir again occasionally and pay attention to settling while adding. If the gel is not sufficiently suspended and the amount of added gel is insufficient, an abnormal value (RLU of B0 < RLU of sample) may result due to insufficient removal of reaction inhibitors. An abnormal value will also result if gel of Sample Pretreatment Solution 2 is mixed in the measurement sample. Also, please be careful not to aspirate the gel when separating the supernatant.
- How should I pretreat highly viscous saliva samples?
- Highly viscous saliva may not yield expected measurement values if it is only pretreated following the procedure specified in the package insert of this kit. In this case, spike the saliva with aprotinin and Proclin™ 950, freeze and thaw the mixture once, and use the supernatant after centrifugation (e.g., 5000 g, 10 minutes, 4°C) as the sample for the pretreatment procedure specified in the package insert of this kit.
- Can I store pretreated samples?
- Storage of pretreated samples is not recommended. If storage is unavoidable, store the sample in a polypropylene or polyethylene tube under refrigeration (2-10°C) and measure it on the following day. Any undissolved matter found at the time of measurement should be removed by centrifugation, etc., and the supernatant should be used as the sample for measurement.
- When I add Sample Buffer 1 to the brain homogenate sample, the mixture becomes turbid. Can I proceed as is?
- Turbidity may occur if the sample has a high protein concentration or contains a lot of cell debris, due to protein denaturation. If, after adding Sample Buffer 2 and centrifuging to remove the gel, you find the supernatant clear of insoluble turbid matter, you can use it as a processed sample for measurement. Should insoluble matter remain, either increase the centrifugal force to around 10,000 g or filter the sample through a 0.2-µm filter before using it as a processed sample.
About analyte
- What is the degree of cross-reactivity with vasopressin?
- Cross-reactivity with [Arg8]-vasopressin is about 0.79%.
- Can mesotocin, isotocin, and vasotocin be measured?
- No data are available for mesotocin, isotocin, and vasotocin.
About the kit
- Is it possible to purchase the antibodies provided in this kit?
- These antibodies are not sold separately.
- How is the standard (oxytocin) of this kit certificated?
- The concentration of oxytocin is certificated using the WHO world standard product (OXYTOCIN 4th International Standard NIBSC code: 76/575).
- Can I divide plates?
- Since the plate in this kit are separable and each row (8 wells) is detachable, divided use of the kit is possible. By detaching the rows containing the portion to be used and store the remainder in a refrigerator. It will remain stable for the shelf life. Use scissors or other tool to cut the necessary portion of the plate seal to cover the wells to be used.
About kit usage
- What reagents, instruments, and equipment are required for the assay using this kit?
- The reagents, instruments, and equipment required for the use of this kit are listed below. This kit uses a highly sensitive competitive ELISA and requires a plate shaker and a plate reader for luminescence measurement.
- Purified water (distilled water)
- Standard solution, tubes for sample dilution
- Glassware for dilution of washing solution (graduated cylinders, beakers)
- Pipettes with disposable tips (disposable tips for 10 µL and 200-500 µL)
- Repeating pipette to dispense 100 µL
- Paper towel or other absorbent material (to remove liquid left on the plate after washing)
- Mixer (Vortex type)
- Microplate shaker (about 600-800 rpm)
- Plate washer for 96-well plates (preferred) or wash bottles
- Plate reader for 96-well plates (for luminescence measurement)
- Software for data analysis
- What type of plate readers can be used?
- A plate reader for luminescence measurement is required for this kit.
- Please let me know the measurement parameters for the plate reader?
- As an example, the parameters when using Infinite M200 PRO (Tecan) are listed below.
Equipment: Infinite M200 PRO (Tecan)
Software: Plate Manager V5
Measurement type: Endpoint measurement
Measurement mode: Emission intensity
Plate size: 12 x 8
Well scan size: 1 x 1
Number of wavelengths: 1
Conversion of measurement values: W1
Units of measurement values: RLU
Attenuation 1: AUTOMATIC
Integration time 1: 500 ms
Settle time 1: 300 ms
- What type of plate shaker should be used?
- A capacity to shake the ELISA plates at about 500 rpm is required.
- Is it possible to provide contract services using this kit?
- You can provide contract services using this kit.
Trouble Shooting
- What should I do if the calibration curve is not good?
- Competitive reactions are reversible, but if it takes too long to add standards or samples after the addition of biotinylated oxytocin solution, the desired calibration curve slope may not be achieved due to increased variability or base emission intensity. As a rough standard, addition of samples or standards should be completed within 10-15 minutes (within approximately 1/10 of the competitive reaction time). If the number of samples is large, it is efficient to dispense the samples into flexible plates, etc. and transfer the musing a multi-channel pipette.
- What should I do if the measurement value of the sample is higher than that of the background?
- A higher RLU value for a sample than that of background may occur when reaction inhibitors remain in the sample after pretreatment(RLU of B0 < RLU of sample in competitive ELISA). In particular, reaction inhibitors may remain if the amount of gel contained in Sample Pretreatment Solution 2 is insufficient. As the gel settles over time after stirring, stir again occasionally and pay attention to settling while adding. An abnormal value will also result if gel of Sample Pretreatment Solution 2 is mixed in the pretreated sample. Also, please be careful not to aspirate the gel when separating the supernatant.
- What should I do if a low concentration range cannot be measured well?
- Perform sufficient stirring in each step, and pay special attention to the following steps:
- Immediately after addition of samples or standards
Sufficient stirring is required. More homogeneous mixing can be achieved by stopping and starting the stirring intermittently as compared with continuous stirring. As a rough standard, it is recommended to perform the procedure [stirring at 600-800 rpm for 10 seconds with a microplate shaker → stopping once → stirring again; 3 times]. - During competitive reaction
A stirring speed of 500 rpm is recommended. Insufficient stirring speed or intensity may result in decreased reactivity in a low concentration range. - Immediately after addition of peroxidase-conjugated streptavidin solution
Sufficient stirring is required in the same manner as for immediately after the addition of samples or standards. As a rough standard, it is recommended to perform the procedure [stirring at 600-800 rpm for 10seconds with a microplate shaker → stopping once → stirring again; 3 times].
- Immediately after addition of samples or standards
- What should I do if bubbles remain in wells during cleaning?
- For manual cleaning using a wash bottle, etc., try the method to avoid bubbles by overflowing the cleaning fluid only in the last round of cleaning. Use the cleaning fluid provided with the kit. Do not use ultrapure water for cleaning. Ultrapure water is also called "hungry water": since it has a strong propensity to incorporate materials coming in contact with it, there is concern about effects on antigen-antibody conjugates or other materials.
- What should I do if the luminous intensity is too high?
- Please check the following two points
- Is it taking a long time after the addition of biotin-labeled oxytocin solution before the next standard solution or sample is added?
- Is the luminescent reagent allowed to warm up to room temperature before use? If the above does not improve the situation, consider shortening the integration time (measurement time) or using an attenuation filter.
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.