Internal Standard for qNMR (Calibration Standard for qNMR)

The requirements for a calibration standard for quantitative NMR (qNMR) are as follows:
- Mass fluctuation due to moisture absorption and sublimation is small and stable weighing is possible.
- Traceability of purity value is guaranteed.
- Giving a chemical shift and showing a simple shape (preferably a single peak). It is also important to select an appropriate calibration standard according to the analysis sample and deuterium solvent1).
FUJIFILM Wako has a lineup of optimal products as calibration standard for qNMR.
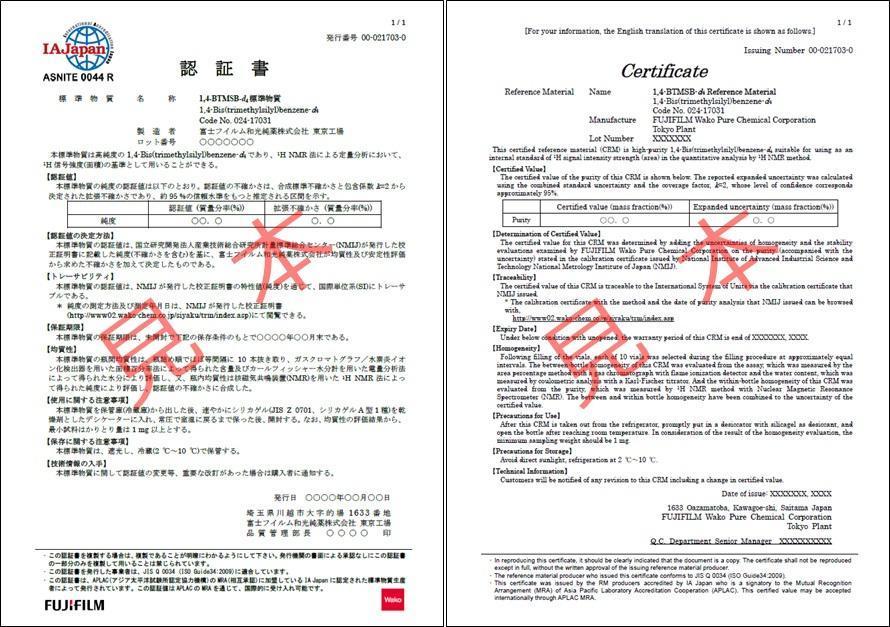
TraceSure® (CRM)
TraceSure® is a CRM series with a certificate in which NMIJ has determined the purity by adding the uncertainty obtained from FUJIFILM Wako's homogeneity and stability evaluation to the purity valued by the SI traceable measurement method. The characteristic values of TraceSure® are SI traceable via the NMIJ analysis values, and can express the measurement traceability. TraceSure® series are accredited as reference material producers by the ASNITE accreditation program operated by IA Japan. The accredited values listed in the TraceSure® series certifications can be accepted internationally by the MRA of ILAC/APAC.
Calibration Standard for qNMR
It is a calibration standard product that uses TraceSure® (CRM) to give purity value by qNMR and measurement uncertainty. Compared to CRM, it is a large-capacity and inexpensive.
Calibration Standard Solution for qNMR
It is a calibration standard prepared by CRM used in qNMR with a deutrium solvent. This product is given the characteristic value by qNMR and the uncertainty which is the proof of reliability. In the sample preparation of the calibration standard solution, it is required to accurately collect the internal calibration standard with a relatively strong signal strength of 1H, so a highly accurate balance such as an ultra-micro balance and a preparation environment are required. Since this calibration standard solution is a pre-prepared, it does not require precise weighing of calibration standard. It can also be used as a calibration standard in the external standard method.
*Outline of internal standard method using calibration standard solution for qNMR.
NMIJ CRM
NMIJ CRM is a certified reference material distributed by NMIJ. Produced by a management system based on ISO17034, and it is SI traceable. In recent years, research on quantification methods using other nuclei has been underway.2.3,4) This product can be used to calibrate the signal strength of 1H and 19F in qNMR, and to confirm the validity of the analysis method.
- "qNMR Primary Guide" Working Group: (2015) qNMR Primary Guide from Basics to Practice", Kyoritsu Shuppan.
- Naoki S et al.: Measurement and control, 53-6, 529 (2014).
- S. Al-Deen et al.: Anal. chim. acta, 474-1-2, 125 (2002).
- He et al.: J. fluorine chem, 127-6, 809 (2006).
Features
When selecting an internal calibration standard, it is important that (1) the compound to be measured and the NMR signal do not overlap, and (2) solubility in the deuterium solvent used. Below is a summary of our calibration standard 1H NMR chart*1 and solubility information. Please use the calibration standard that matches the compound to be measured.
*1 The 1H NMR chart may change slightly depending on the conditions.
-
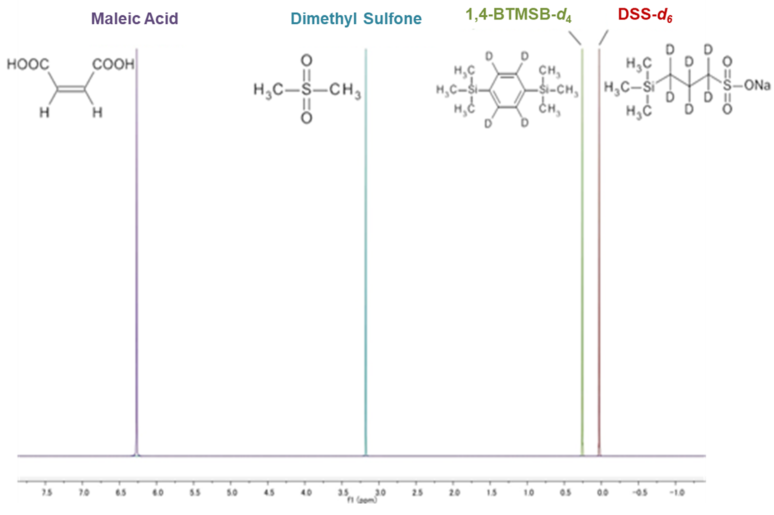
TraceSure® (CRM)
-
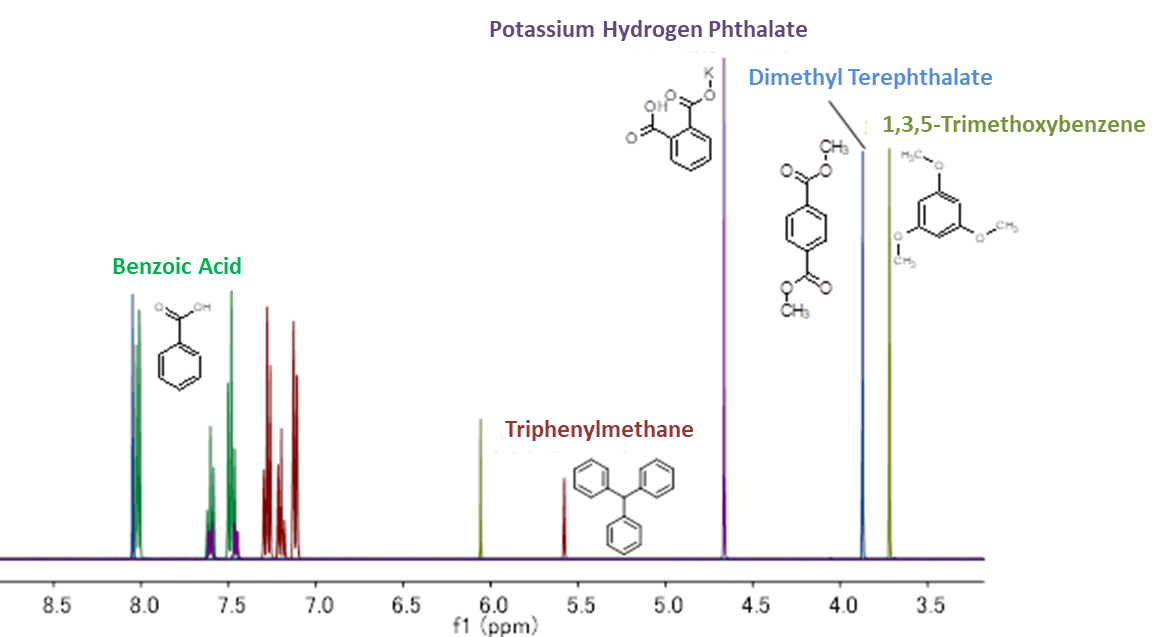
Calibration standard for qNMR
Solubility Information
| TraceSure® (CRM) | Calibration standard for qNMR | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Solvent/ Standard | Maleic Acid | Dimethyl Sulfone | 1,4-BTMSB-d4 | DSS-d6 | Benzoic Acid | Triphenylmethane | Potassium Hydrogen Phthalate | Dimethyl Terephthalate | 1,3,5-Trimethoxybenzene |
| Acetone-d6 | 〇 | 〇 | 〇 | ✖ | 〇 | 〇 | ✖ | 〇 | 〇 |
| CDCl3 | ✖ | 〇 | 〇 | ✖ | 〇 | 〇 | ✖ | 〇 | 〇 |
| DMSO-d6 | 〇 | 〇 | △ | 〇 | 〇 | 〇 | △ | △ | 〇 |
| CD3OD | 〇 | 〇 | 〇 | 〇 | 〇 | ✖ | ✖ | ✖ | 〇 |
| D2O | 〇 | 〇 | ✖ | 〇 | ✖ | ✖ | 〇 | ✖ | ✖ |
| CD2Cl2 | ✖ | 〇 | 〇 | ✖ | 〇 | 〇 | ✖ | 〇 | 〇 |
〇: Soluble △: Slightly soluble ✖: Insoluble
Cautionary Points When Selecting the Calibration Standard
The quality of the calibration standard is one of the most important components in the qNMR measurement. The reason why qNMR technique is beginning to be used in many fields including pharmaceuticals and food analysis is that it can obtain accurate quantitative results with comparative ease as compared with other conventional techniques by utilizing calibration standard with highly reliable assigned purity or concentration (e.g., calibration standards that ensure SI traceability). In other words, this characteristic of qNMR means that it is extremely important to select the appropriate calibration standard.
What are the cautionary points when selecting the calibration standard used for qNMR?1,2)
- Signal (signals) with a specific chemical shift where a signal of general organic compound is not observed
- Good solubility in solvent
- Accurate weighing is possible (i.e., non-hygroscopic, non-deliquescent or non-volatile / non-sublimable property)
- Less signals and less spin-spin coupling (singlet is preferred)
- With traceability to the International System of Units (SI) (e.g., National Standard, Certified Reference Material (CRM), etc.)
So far we have exemplified the main points for selecting a calibration standard used for qNMR. In qNMR measurement, it will be possible to obtain a quantitative value with SI traceability (absolute quantitative value) by using a calibration standard with SI traceability.
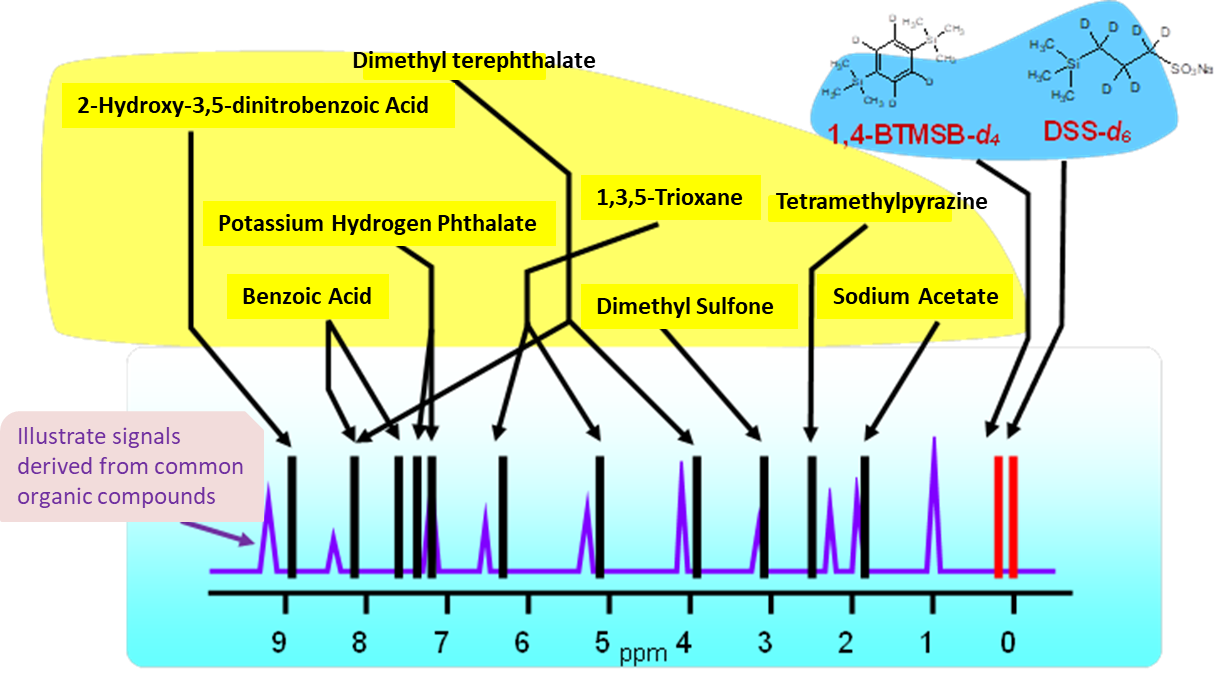
1,4-BTMSB-d4 certified reference material (CRM) and DSS-d6 CRM are calibration standard for 1H qNMR that satisfy the above points. These two CRMs satisfy all of the above points, and because of their high versatility, have been adopted by the purity value by quantitative NMR (qNMR) for multiple compounds in the Japanese Pharmacopoeia. In the figure above, the chemical shifts of general qNMR calibration standards reported in scientific papers and general organic compounds are schematically shown. It can be seen that the 1,4-BTMSB-d4 and the DSS-d6 give a singlet signal around 0 ppm, and have a higher ability to separate from general organic compounds than other general calibration standards.3), 4). In addition, the 1,4-BTMSB-d4 has good solubility in organic solvents, and the DSS-d6 has good solubility in aqueous solvents.
- JIS K 0138: 2018
- Miura T. et al., Analytical standards purity determination using quantitative nuclear magnetic resonance. New Horizons of Process Chemistry: Scalable Reactions and Technologies. (Tomioka K. et al.). Springer, Singapore, 275 (2017).
- Wako Junyaku Jiho, Vol. 86 No. 1 (January 2018)
- Inspired by Dr. Naoki Sugimoto at NIHS.
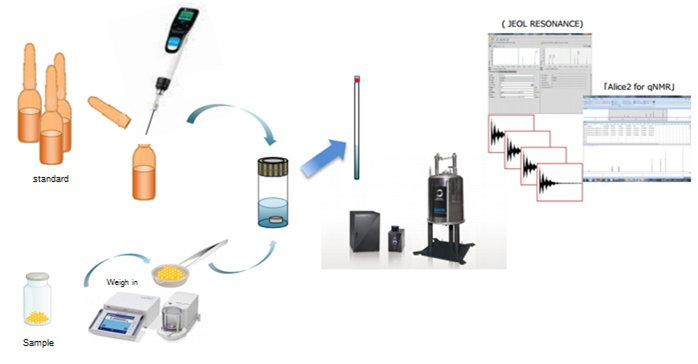
Weighing Procedure for qNMR
In the purity value by quantitative NMR (qNMR) stipulated in the Reagents / Test Solutions section of the Japanese Pharmacopoeia, the weighing is performed using an ultra-micro balance. However only small items, such as a small vial, can be placed since the weighing pan of the ultra-micro balance is very small. In addition, it is very difficult to place this small vial on a weighing pan, tare it, and then put the sample into the vial with a spatula without adhering it to the edge of the vial. In some cases, the sample may adhere to the outside of the vial or to the weighing pan of the balance, which can be unrecognized and cause large errors in the measurement results. From the above, the procedure of using a small metal weighing dish such as made of aluminum as a tare is widely utilized as a method of performing accurate weighing with an ultra-micro balance. This procedure is shown in the figure below. By using this method, the sample and the calibration standard for qNMR can be skillfully placed in a container such as a vial without adhering to the edge of the vial or the weighing pan of the balance.
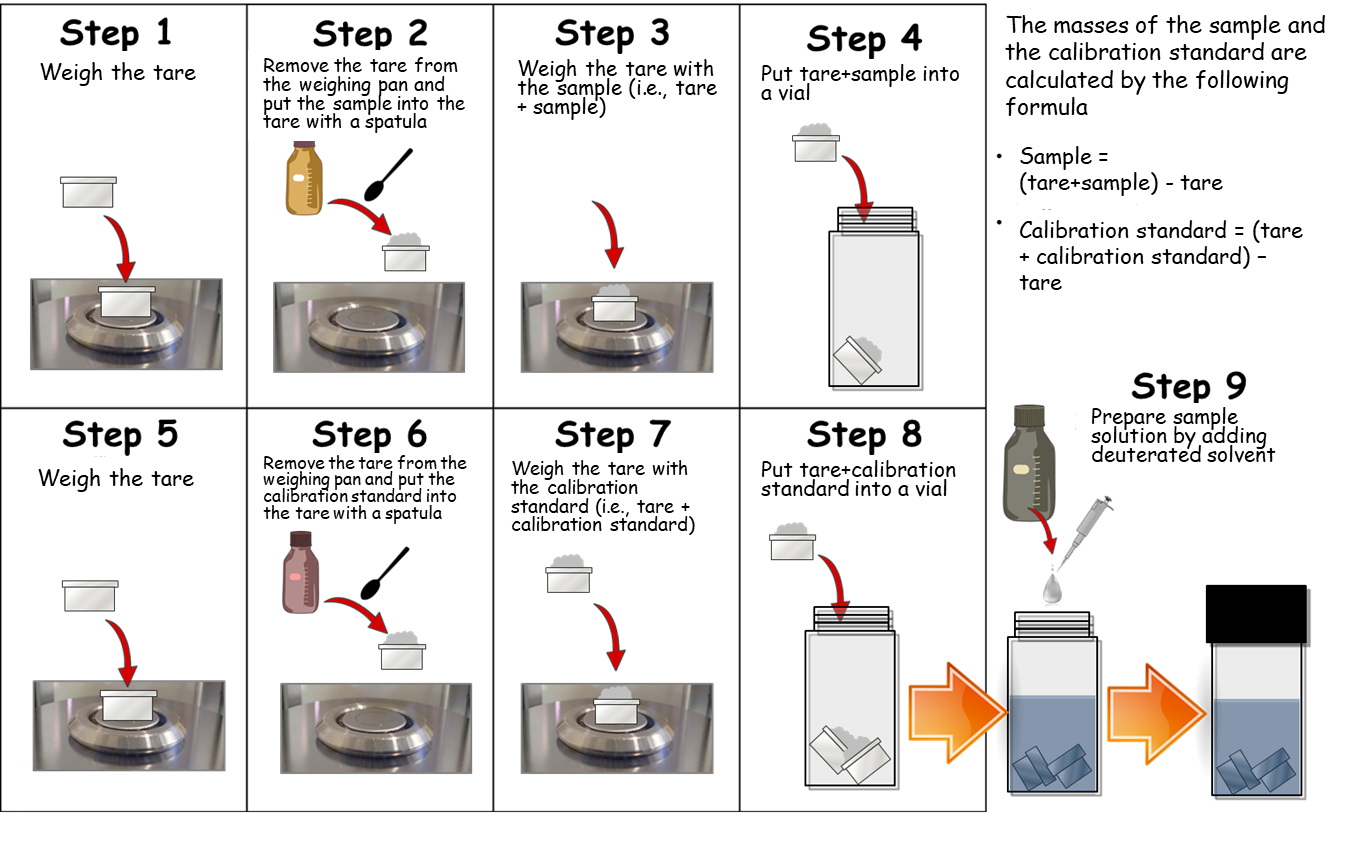
Here, we would like to introduce an aluminum weighing dish as the most suitable tare for weighing in qNMR measurement. The aluminum weighing dish is made of aluminum and is not easily electrostatically charged. It has a small volume and is not easily affected by buoyancy effect in the air, so it is suitable for accurate weighing in qNMR measurement. In addition, it has an appropriate internal volume (80 μL) for performing qNMR measurement. Furthermore, elution tests were conducted on 7 types of deuterated solvents (chloroform-d, DMSO-d6, deuterium oxide, acetone-d6, acetonitrile-d3, methanol-d4 and dichloromethane-d2), and confirmed that there are no signals from eluates.
We also offer wide-mouthed quartz vials suitable for loading aluminum weighing dish.
Minimum Weight
Minimum Weight is the minimum sample quantity required for an accurate weighing of the mass of the sample with the measurement error of the balance used taken into account.
In the pharmaceutical industry, the United States Pharmacopeia (USP) General Chapter <41>; Balances and General Information <1251>; Weighing on an Analytical Balances are widely utilized as one of the methods for the control of balances 1,2). In order to weigh the sample accurately on the balance, the balance must be calibrated within its range of utilization and meet the following criteria:
- Repeatability is assessed by weighing one test weight not less than 10 times.
- Repeatability is satisfactory if two times the standard deviation of the weighed value, divided by the desired smallest net weight (i.e., smallest net weight that users plan to use on that balance), does not exceed 0.1%.
In other words, the following equation is derived.

An example of assessing the Minimum Weight is shown below. As you can see from this example, in order to ensure the same accuracy from difference readability (scale interval) of balances, you can see that the smaller readability (scale interval) of the balance, the smaller amount of the sample can be weighed.
Measurement example of Minimum Weight
| Type of balance | Minimum Weight (Wmin) |
|---|---|
| Semi-micro balance (readability: 0.01 mg) | 13.9 mg |
| Micro balance (readability: 0.001 mg) | 2.8 mg |
| Ultra-micro balance (readability: 0.0001 mg) | 0.2 mg |
In solution NMR, the sample is dissolved in a deuterated solvent for NMR measurement, but the deuterated solvent used is more expensive than the general solvent used for HPLC and GC, so a lesser amount of solvent is desired. The situation is the same for qNMR measurements, and assuming a fixed concentration, a balance with a smaller readability can save the amount of deuterated solvent used. In fact, the Japanese Pharmacopoeia has adopted the purity determination with qNMR for multiple compounds in the Reagents / Test Solutions section, but all the balances used are specified as ultra-micro balance.*1
*1 The ultra-micro balance in the Japanese Pharmacopoeia refers to a balance with readability (scale interval) of 0.1 μg
- USP "General Chapter 41 Balances", USP39-NF34, cited 29 April, (2016).
- USP "General Information 1251 Weighing on an Analytical Balance", USP39-NF34, cited 29 April, (2016).
Product List
- Open All
- Close All
TraceSure® (CRM)
Analytical Standard for qNMR (non-CRM)
Standard Solution for qNMR (CRM)
Standard Solution for qNMR (non-CRM)
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



