6th review: Control of stroke via microglia-astrocyte crosstalk
This article was written by Dr. Schuichi Koizumi, Department of Neuropharmacology, Interdisciplinary Graduate School of Medicine, University of Yamanashi.
Introduction
The roles of glial cells in brain function have been successively clarified. Given rapid, substantial, and various changes in glial cells in various brain diseases, it is assumed that these changes in glial cells may play an important role in brain abnormalities and repair processes. While these hypotheses are gradually being accepted, several issues remain to be addressed. For instance, large deviation between in vitro and in vivo results in glial cell research makes it difficult to extrapolate exclusively in vitro experimental results to the in vivo situation. In addition, many studies have focused on only one glial cell population such as microglia, astrocytes, or oligodendrocytes without recognizing that brain function is regulated through communication among different glial cell populations. Due to the relative newness of and little experience in glial cell research, it is of course very important to characterize each glial cell population at first, whereas recent studies have suggested in various forms that glial cells may regulate brain function through collaboration among different glial cell populations as an assembly. In this article, focus is placed on microglia-astrocyte crosstalk in an in vivo stroke model to describe the importance of communication in the regulation of brain function.
Microglia-astrocyte crosstalk
Microglia are very sensitive to changes in the environment inside and outside the brain and change themselves in response to any subtle change in advance of brain changes. For instance, microglia are already activated in middle age prior to aging1) and involved in the pathogenesis and refractoriness of neurodegenerative diseases such as amyotrophic lateral sclerosis by being activated before the onset.2) Activated microglia not only regulate neuronal function directly, but also act on astrocytes to affect brain function. In a model of traumatic brain injury, microglia first sense the injury and communicate it to astrocytes to protect nerve cells.3) When sensing inflammation, microglia communicate it to astrocytes to regulate neuronal function.4)
Ischemic tolerance, a phenomenon where noninvasive ischemic stress is followed by the development of resistance to subsequent invasive ischemia, is well known both experimentally and clinically (Fig. A and B). In a mouse model of transient middle cerebral artery occlusion (MCAO), notable changes occur initially in microglia and then in astrocytes.5) Even noninvasive MCAO (preconditioning, PC), which causes no significant damage, activates sensitive microglia. Ischemic cross-tolerance, a phenomenon where ischemic tolerance is induced by PC other than noninvasive MCAO, is also known, and the representative PC that induces ischemic cross-tolerance is lipopolysaccharide (LPS), a cell wall component of gram-negative bacteria. LPS acts strongly on Toll-like receptor 4 in microglia in the central nervous system, suggesting that microglia are essential for the induction of ischemic tolerance. In these mechanisms underlying microglia-mediated induction of ischemic tolerance, a type I interferon pathway is deeply involved, and microglia-dependent gliotransmitters, cytokines, and neurotrophic factors also play important roles. Microglia induce ischemic tolerance not only directly by acting on nerve cells, but also indirectly by acting on other glial cells such as astrocytes, and the importance of the latter has recently been suggested.
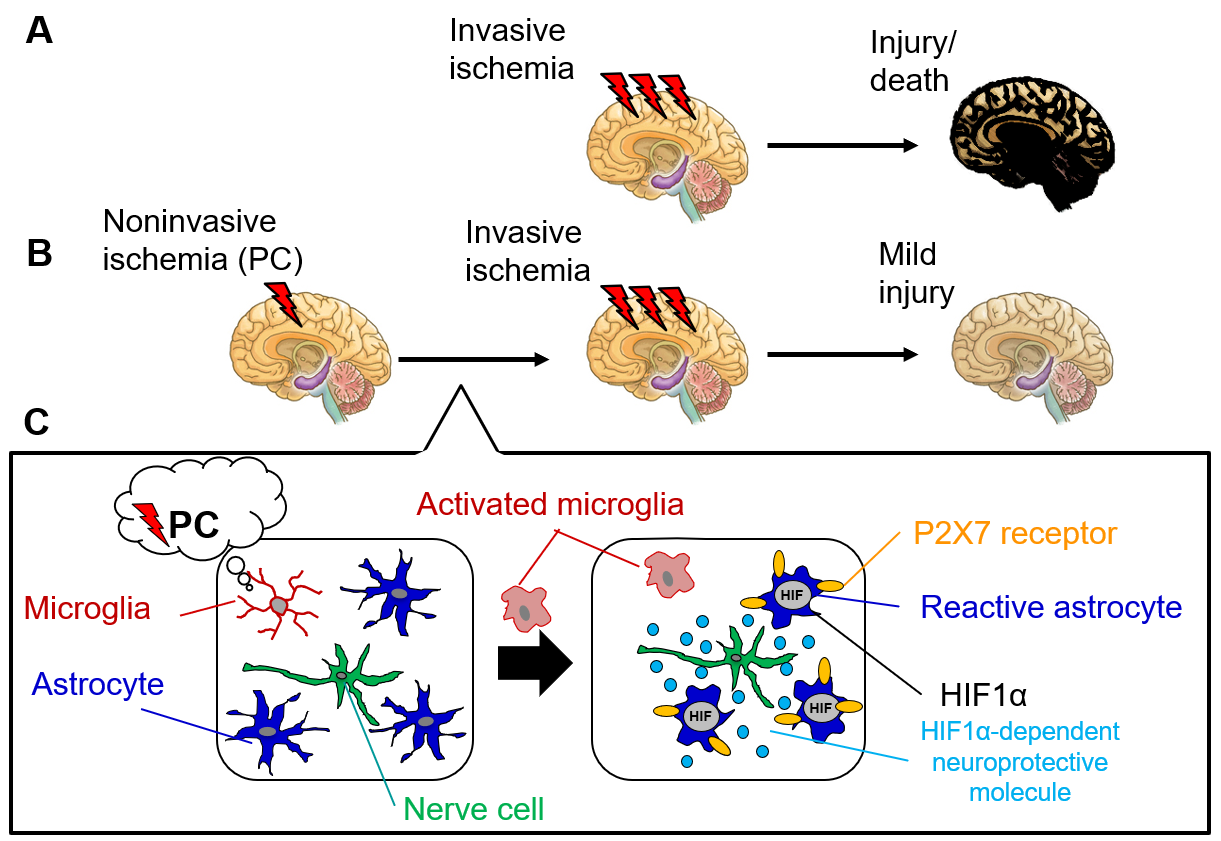
Figure: Mechanisms of ischemic tolerance acquisition through glial communication
A. Invasive cerebral ischemia results in severe neuronal injury or death.
B. Noninvasive ischemia (PC) induces ischemic tolerance, an acquired resistance to invasive ischemia.
C. Mild stimuli such as PC are first sensed by microglia. The microglia are activated not only to be directly involved in the induction of ischemic tolerance, but also to activate astrocytes (reactive astrocytes). Reactive astrocytes contribute to the development of ischemic tolerance through multiple mechanisms, and neuroprotective mechanisms through P2X7 receptor expression and P2X7 receptor-dependent HIF1α expression are known to result in particularly durable and robust ischemic tolerance.
Signaling from microglia to astrocytes
How do microglia sensing PC send the information to astrocytes? Among various known damage-associated molecular patterns (DAMPs), adenosine triphosphate (ATP) is an important initial signal. Initial changes in microglial response during early stroke and under LPS stress include significant changes in ATP/P2 receptor signaling. Upon sensing LPS, microglia express the vesicular nucleotide transporter to stimulate the exocytosis of ATP.6) Upon sensing stroke, microglia express the P2Y1 receptor, which is not normally expressed, in order to receive ATP and significantly change their own function.7) In particular, ATP plays a central role as an information signal from microglia to astrocytes in the initial response to highly excitatory stimuli4) or traumatic brain injury,3) in addition to stroke. It is suggested that microglia, although they themselves protect the brain,7) may function as a unique safeguard against various brain diseases through a cascade where microglia transmit their information to astrocytes, which sense the information more sensitively and protect the brain more strongly.
Astrocytes as cells responsible for ischemic tolerance acquisition
PC activates astrocytes (reactive astrocytes). Since inhibition of the activation results in loss of ischemic tolerance, it can be said that reactive astrocytes are requisite for the induction of ischemic tolerance. Reactive astrocytes are responsible for ischemic tolerance acquisition and play a central role in the induction of ischemic tolerance by acting directly or indirectly through communication with nerve cells and other cells. For instance, reactive astrocytes, which have a wide range of effects, including production of neuroprotective molecules, enhanced clearance of excitatory neurotransmitters and toxic substances, and regulation of energy metabolism, strongly act especially through P2X7 receptor.5, 8, 9) PC-activated astrocytes increase the expression of P2X7 receptor significantly (≥100 times) for a long period of time (≥8 weeks) in an astrocyte-specific manner to induce hypoxia-inducible factor1α (HIF1α) in a receptor-dependent manner, resulting in very strong resistance to ischemia (Fig.). Much remains unknown about the mechanisms for inducing astrocyte-mediated ischemic tolerance, including the mechanism of the astrocyte-specific increase in P2X7 receptor expression. Since microglial activation precedes PC-induced astrocyte activation as described above, microglia may induce astrocyte-mediated ischemic tolerance. However, much remains unknown about the right and wrong of the induction and the microglia-induced phenotypic variability in astrocytes, which requires further research. In addition, there are many unanswered questions. For instance, how do microglia sense noninvasive mild PC? Which phenotype do microglia change to? What are the signals from microglia to astrocytes and nerve cells? Nonetheless, the mechanism in which microglia and astrocytes transmit any change in the brain environment in a relay fashion to alter the brain response to it may be a common phenomenon preceding various brain diseases, suggesting the great significance of its ripple effects and thus requiring future research progress.
Conclusion
The importance of glial cells in regulation of brain function is exemplified by ischemic tolerance. While both microglia and astrocytes have the potential to induce ischemic tolerance, microglia-astrocyte crosstalk-mediated interactions may actually play more important roles. Individual glial cell populations have been increasingly studied, but the perspective of such interactions between different cell populations to regulate nerve cells is expected to become more important in the future.
References
- Zhang, G. et al. : Nature, 497, 211 (2013).
- Sanagi, T. et al. : J. Neurosci. Res., 88, 2736 (2010).
- Shinozaki, Y. et al. : Cell Rep., 19, 1151 (2017).
- Pascual, O. et al. : Proc. Natl. Acad. Sci. U S A, 109, E197 (2012).
- Hirayama, Y. et al. : J. Neurosci., 35, 3794 (2015).
- Imura, Y. et al. : Glia, 61, 1320 (2013).
- Fukumoto, Y. et al. : J. Cereb. Blood Flow Metab., 39, 2144 (2019).
- Hirayama, Y. and Koizumi, S. : Glia, 65, 523 (2017).
- Hirayama, Y. and Koizumi, S. : Neurosci. Res., 126, 53 (2018).




