Imaging
Imaging in the life science field is used for the purposes of observing morphology/structure, distribution/localization, dynamics/interactions, etc., and various methods are available depending on the microscope used, observation target, and sample type.
Fujifilm Wako has a large lineup of reagents that support new imaging technologies such as tissue clearing and correlative light and electron microscopy (CLEM), and luminescent/fluorescent probes that are commonly used in imaging.
Product Line-up
More Information
Equipment and Reagents Required for Imaging
Microscope
With a microscope, small objects that are invisible to the naked eye can be magnified and observed. However, the components of cells and tissues have similar light transparency, and it is difficult to recognize their morphology and structure due to this low contrast. Therefore, for observation with a typical optical microscope, the contrast is enhanced by staining or optical processing. There are various other types of microscopes, including fluorescence microscopes and electron microscopes (Table 1).
Table 1: Features and Applications of Representative Microscopes
| Type of Microscope | Feature | Application |
|---|---|---|
| Bright-field microscope | General optical microscope. Illumination brightens the entire field of view. Contrast is enhanced by staining. | Observation of stained specimens |
| Dark-field microscope | Light is illuminated from the side of the specimen and the scattered light is observed. | Observation of unstained specimens |
| Phase-contrast microscope | The phase differences between diffracted light and direct light generated by the differences in refractive index of the specimen are converted to light and dark features to generate contrast for observation. | Observation of colorless and transparent specimens |
| Differential interference microscope | The gradients of optical path length (refractive index x thickness) in the specimen are converted to light and dark features to generate contrast for observation. | Observation of colorless and transparent specimens |
| Fluorescence microscope | Excitation light is irradiated to a fluorescent substance in the specimen, and the emitted fluorescence is processed for imaging. | Observation of cells and tissues labeled with fluorescent dyes |
| Confocal microscope | A point light source and pinhole allow focusing within the specimen to obtain non-invasive sectional images at high resolution. | Three-dimensional observation of cells and tissues (often labeled with fluorescent dyes) |
| Scanning electron microscope | The specimen is scanned with an electron beam, and the images obtained from the secondary and backscattered electrons are observed. | Observation of microstructures on the surface of the specimen |
| Transmission electron microscope | An electron beam is applied to the specimen, and the image obtained from the transmitted electrons is observed. | Observation of microstructures inside the specimen |
[Featured products] Reagents for correlative light and electron microscopy (CLEM)
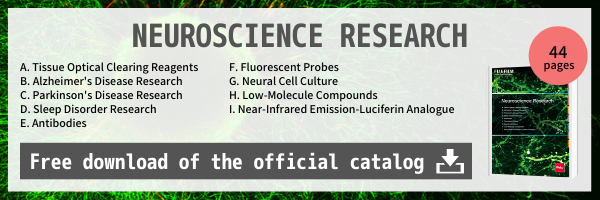
Correlative light-electron microscopy (CLEM) is a technique for analyzing the localization and morphology of intracellular organelles/cellular molecules by observing the same section under both light and electron microscopes.
With conventional methods, it was difficult to perform optical microscopy and electron microscopy on the same section due to attenuation of fluorescent dye. Fujifilm Wako’s fluorescence recovery reagents allow for imaging of the same section.
Pretreatment Reagents
When observing cells and tissues under a microscope, the original state of the specimen must be fixed and preserved. Fixation is generally performed by cross-linking biomolecules using formalin solution or other reagents to immobilize the specimen and prevent degradation/decomposition. When antibodies are used to detect proteins, specimens are treated with a detergent (Triton X-100) or organic solvent to permeabilize the plasma membrane. Other processing includes removal of unnecessary staining reagents/probes and blocking to prevent nonspecific binding of antibodies, depending on the experimental system.
[Featured products] Tissue Clearing Reagents
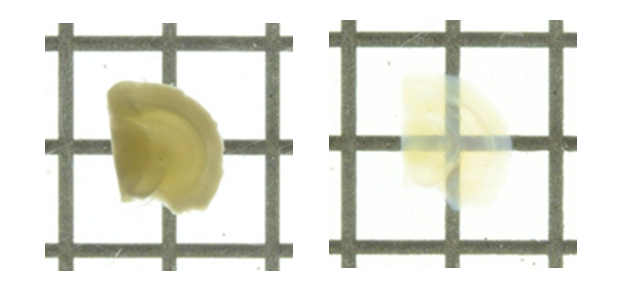
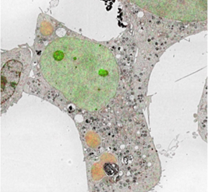
When analyzing biological tissues, light scattering occurs due to inclusion of various materials with different refractive indices.
To make a biological tissue transparent, the refractive index of the tissue needs to be made uniform by removing components with high-refractive index from the tissue, replacing the solvent with a liquid with high-refractive index, or performing both.
Clearing the tissue allows for deep tissue imaging and high-resolution observation.
Probe
To observe specific cells/molecules and biological phenomena, probes that are specific for the target should be selected. Below are descriptions of luminescent/fluorescent probes, which are frequently used for imaging.
Luminescent Probes
Luminescent probes are excited by a chemical reaction and then emit light as they return to the ground state. Because luminescence depends on the chemical reaction, the technique is highly selective, has low noise, and produces a strong signal. It offers a wide dynamic range and is often used to analyze the dynamics of ATP and calcium ions.
Typical luminescent probes include firefly luciferin-luciferase (FLuc), apoaequorin, or Renilla luciferase (RLuc)-coelenterazine.
Fluorescent Probes
Fluorescent probes are first excited by light and then re-emit the light as they return to the ground state. Unlike luminescent probes, fluorescent probes have the advantage of requiring no reaction substrate; a signal can be obtained by simply applying excitation light. On the other hand, fluorescent probes have the disadvantages of having higher noise due to fluorescent substances other than the fluorescent probes in living organisms, and a narrower dynamic range compared to luminescent probes.
Commonly used fluorescent probes include organic fluorescent dyes, such as rhodamine and fluorescein, and fluorescent proteins such as GFP. Fujifilm Wako offers a wide variety of probes that specifically label cells and organelles, as well as probes used for functional analysis (Table 2).
Table 2: Fluorescent Probes by Subject of Observation
| Subject of Observation | Target and Fluorescent Probe |
|---|---|
| Cell morphology and movement | Cytoskeleton (actin filaments, microtubules, intermediate filaments) Phalloidin binds specifically to actin filaments, and labeled phalloidin is used as a probe. Other frequently used probes include antibodies specific for cytoskeletal proteins and fusion proteins between a fluorescent protein and cytoskeletal protein. |
| Specific cells/organelles | Nucleus Stained with DNA-specific fluorescent dye such as DAPI, bisbenzimide (Hoechst) 33258/33342, and propidium iodide. Nucleolus With RNA-specific fluorescent dye, intense fluorescence can be observed in the nucleolus, which is the site of rRNA production. Mitochondria Stained with a membrane potential-dependent fluorescent reagent taking advantage of the membrane potential generated by a proton gradient during energy production. Rhodamine123 and JC-1 are commonly used, but products with improved cellular retention and cytotoxicity are also available. Endoplasmic reticulum and Golgi apparatus Fluorescent lipid derivatives are versatile probes. Fusion proteins between a fluorescent protein and protein localized to the endoplasmic reticulum or Golgi apparatus are also used. Neurons (neural circuits) Neurons and neuronal circuits can be labeled by injection into neurons. Dyes that are injected into the cell body to label the nerve endings are called “anterograde tracers,” while dyes that are injected into the nerve endings to label the cell body is called “retrograde tracers.” Examples include carbocyanine dyes and dextran gold. |
| Localization of biomolecules | Nucleic acid Nucleic acids complementary to target nucleic acids are used as probes. Probes are labeled with FITC or Cy5. Proteins Antibodies are commonly used. The primary antibody can be directly linked to a fluorophore, or a fluorescent-labeled secondary antibody is used for detection. If appropriate antibodies are not available, the protein of interest can be tagged with an affinity tag or a fluorescent protein and expressed. Glycans Lectins that bind specifically to glycans are fluorescent-labeled and used for detection. |
| Intracellular activity | Intracellular signal transduction Intracellular signaling involving calcium can be observed using calcium-sensitive probes, of which the fluorescence intensity changes in response to calcium concentration. Calcium probes include aequorin, Indo-1, Fluo-4, and Fura-2. pH changes Fusion with lysosomes and degradation of contents during autophagy can be detected using pH-sensitive probes, of which the fluorescence intensity changes in response to pH. Representative pH-sensitive fluorescent probes include BCECF and SNARF-1 |
| Interactions | Protein interactions Interaction of two proteins can be analyzed using the principle of fluorescent resonance energy transfer (FRET). The two proteins are labeled with different fluorescent probes (e.g., CFP and YFP), in which the fluorescence of one excites the other when the two come into close proximity. |
References
- “GFP and Bioimaging” ed. by Miyawaki, A., Yodosha, Japan, (2000). (Japanese)
- "How to select and use fluorescent and luminescent reagents for successful experiments” ed. by Miwa, Y., Yodosha, Japan, (2007). (Japanese)
- “Handbook for Staining and Bioimaging Experiments 5th ed.” ed. by Takata, K., Saito, N. and Kawakami, H., Yodosha, Japan, (2012). (Japanese)
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.
The prices are list prices in Japan.Please contact your local distributor for your retail price in your region.