Diltiazem Hydrochloride
- for Biochemistry
- Specification Assay :
- 98.0+% (Titration)
- Manufacturer :
- FUJIFILM Wako Pure Chemical Corporation
- Storage Condition :
- Keep at 2-10 degrees C. (RT)
- CAS RN® :
- 33286-22-5
- Molecular Formula :
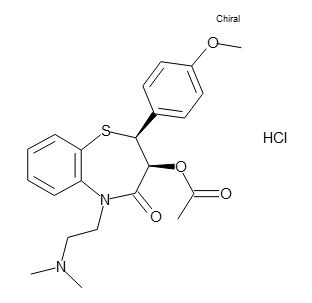
- C22H26N2O4S・HCl
- Molecular Weight :
- 450.98
- GHS :
-
- Structural Formula
- Label
- Packing
- SDS
|
Comparison
|
Product Number
|
Package Size
|
Price
|
Availability
|
Certificate of Analysis
|
Purchase |
|---|---|---|---|---|---|---|
|
|
|
1G
|
|
In stock in Japan |
||
|
|
|
5g
|
|
Discontinued
|
※Check availability in the US with the distributor.
Document
Application
Overview / Applications
| Outline | <Pharmacologic and Physiologic Research><Cardiovascular Active Substance>The heart has automaticity due to the conducting system, and is also influenced by autonomic nerves. The sympathetic nerve acts in an exciting manner, and the parasympathetic nerve in a suppressive manner.Calcium antagonistIt is widely used as a therapeutic substance for circulatory diseases such as hypertension, angina and arrhythmia. It inhibits voltage-dependent calcium channels and reduces the inflow of Ca2+ from extracellular to intracellular parts. Specific calcium antagonists include nifedipine, verapamil and diltiazem.<Cellular Research><Calcium Channel Inhibitor> |
|---|
Property
| Appearance | White, crystals - powder |
|---|---|
| pH | pH (10g/l, 25 degrees C) : 4.3 - 5.3 |
| Melting Point | about 210 degrees C |
| Specific Rotation | [α]D20 +114 - +120° (c=1, H2O) |
Manufacturer Information
Alias
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.