Standard Solution for Volumetric Analysis
Volumetric analysis is a method of quantitative (titration) based on the volume of a standard solution added to a sample solution at the point when it reacts without excess or deficiency. In order to perform accurate quantitative analysis, the concentration value of the standard solution must be accurate. Fujifilm Wako has a variety of certified standard solutions (CRM) prepared and standardized in accordance with the Japanese Pharmacopoeia and CRM conforming to JIS K 8005 that can be used for volumetric analysis.
Usefulness of Reliable Reference Standards for Values
When measuring the size of an object by a yardstick, the accuracy of the measured value depends on the accuracy of the value of the yardstick.
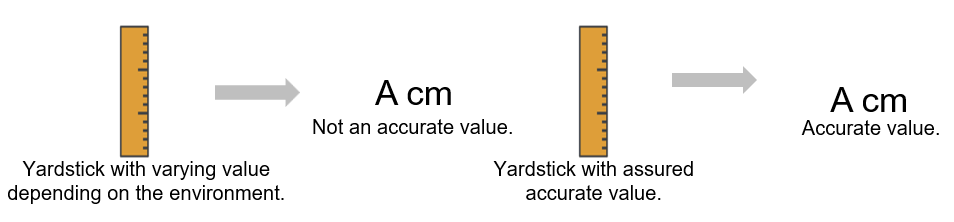
In the case of volumetric analysis, it is the standard solution that serves as the yardstick. Quantitative value measured with standard solution that are assured to have accurate concentration/purity value is highly reliable. Accuracy of the standard solution has indicators such as SI traceability being ensured, proper preparation procedure, etc.
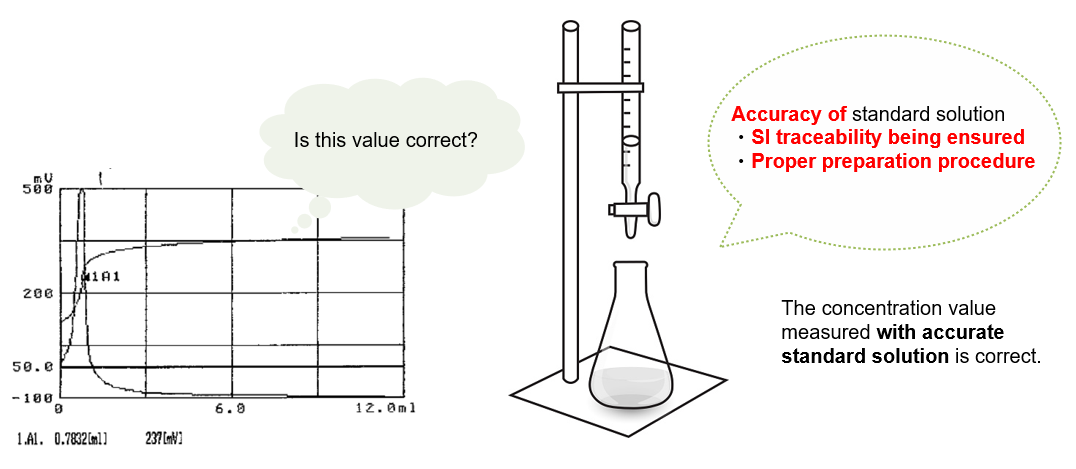
What is SI traceable?
SI traceable means that the concentration or purity value of a substance can be determined using a CRM and the result can be traced back to SI units through the certified value listed on the certificate attached the CRM. SI traceable measurement value is universal and reliable.
Lineup of Analytical Standards for Volumetric Analysis
for the Japanese Pharmacopoeia General Tests
- CRM with assured SI traceable concentration value (factor)! (according to the ASNITE accreditation program)*
- Standard solutions prepared and standardized according to the Japanese Pharmacopoeia.
- Issue a certificate with the accreditation symbol (listed on concentration value, uncertainty, etc.)!
*Some products are not CRM.
TraceSure®

- CRM with assured SI traceable certified value! (according to the ASNITE accreditation program)
- Reference material conforming to JIS K 8005.
- Issue a certificate with the accreditation symbol (listed on purity, uncertainty, etc.)!
Differences in grades of analytical standards for volumetric analysis
| for the Japanese Pharmacopoeia General Tests [CRM] | for the Japanese Pharmacopoeia General Tests | TraceSureⓇ | for Volumetric analysis*1 | |
|---|---|---|---|---|
| Prepared and standardized according to the Japanese Pharmacopoeia 18th Edition | ✔ | ✔ | -*2 | - |
| CRM (SI traceability) | ✔ | - | ✔ | - |
| Attachment of certificate | ✔*3 | - | ✔ | - |
*1: This product is a standard solution assured by Fujifilm Wako.
*2: TraceSure® is a single compound, not a prepared solution (We do not offer custom-made preparation).
*3: Please contact us or our distributor for a certificate.
Certificate

*The certificate is in Japanese. Please contact us if you need the English version.
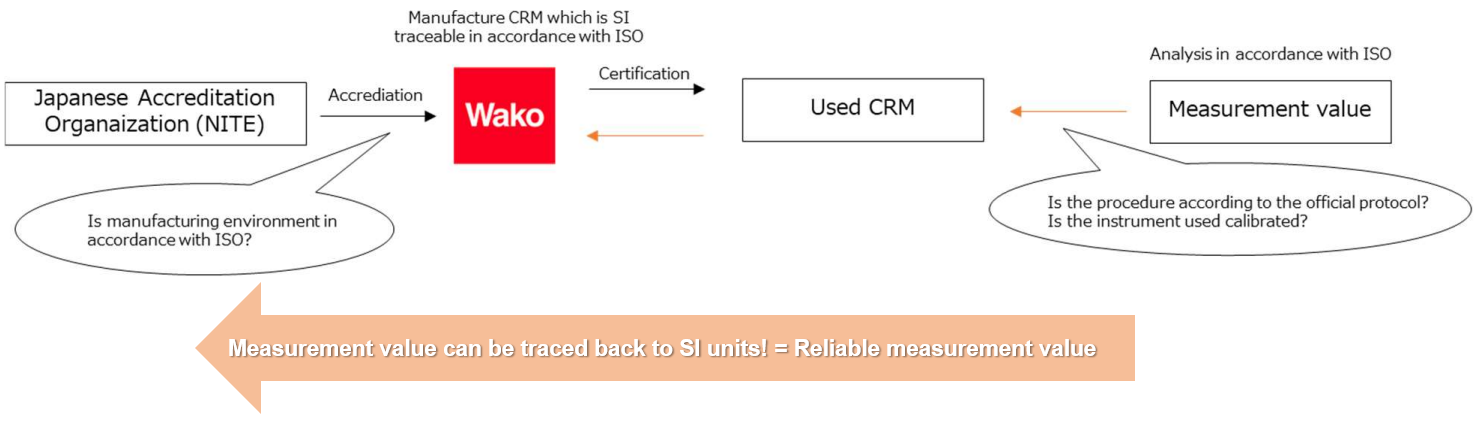
Fujifilm Wako has been accredited as a reference material (RM) producer and supply CRM under the Accreditation System of National Institute of Technology and Evaluation (ASNITE) accreditation program operated by the International Accreditation Japan (IAJapan) of National Institute of Technology and Evaluation (NITE) for volumetric analysis standard solution (for the Japanese Pharmacopoeia General Tests).
CRM for the Japanese Pharmacopoeia General Tests are prepared and standardized in accordance with the Japanese Pharmacopoeia to assure their concentration values, and a certificate with the IAJapan accreditation symbol is issued to certify this.
The following information is listed in the certificate;
- Product name and Code No.
- Lot No.
- Expiration date
- Homogeneity
- Certified value and uncertainty
- Traceability source
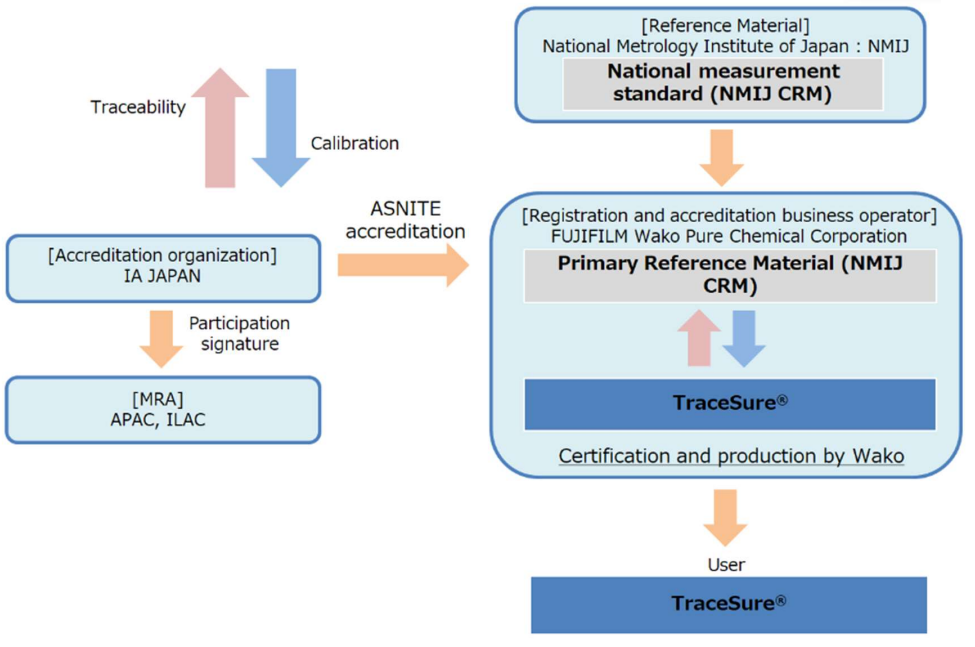
SI Traceability Mechanism
TraceSure®
TraceSure® for Volumetric Analysis is a series of CRM whose characteristic values (purity) are determined by adding uncertainties obtained from our homogeneity and stability evaluations to the value measured with the NMIJ CRM as primary RM. The characteristic value of these RMs are traceable to SI through the NMIJ CRM.
The certified values listed on the certificate of TraceSure® series are internationally acceptable. Fujifilm Wako is the RM producers accredited through the ASNITE accreditation program operated by IA Japan. It means that the certified values listed on the certificate of TraceSure® series are internationally acceptable through APAC's MRA.
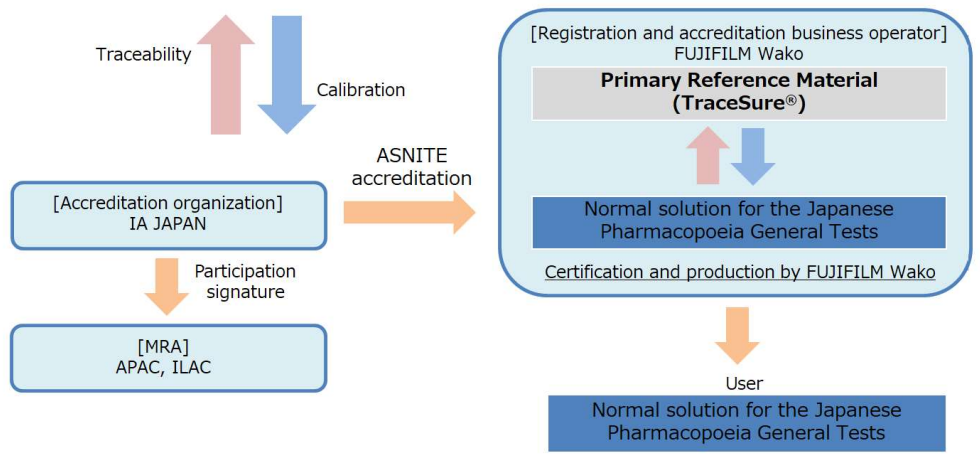
for the Japanese Pharmacopoeia General Tests [CRM]
Standard solution for the Japanese Pharmacopoeia General Tests is a CRM traceable to TraceSureⓇ. This series is prepared and standardized in accordance with the Japanese Pharmacopoeia.
Product List
- Open All
- Close All
for the Japanese Pharmacopoeia General Tests [CRM]
for the Japanese Pharmacopoeia General Tests (non-CRM)
TraceSure® (CRM)
for Volumetric Analysis (non-CRM)
for Colloidal Titration
Indicator
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



