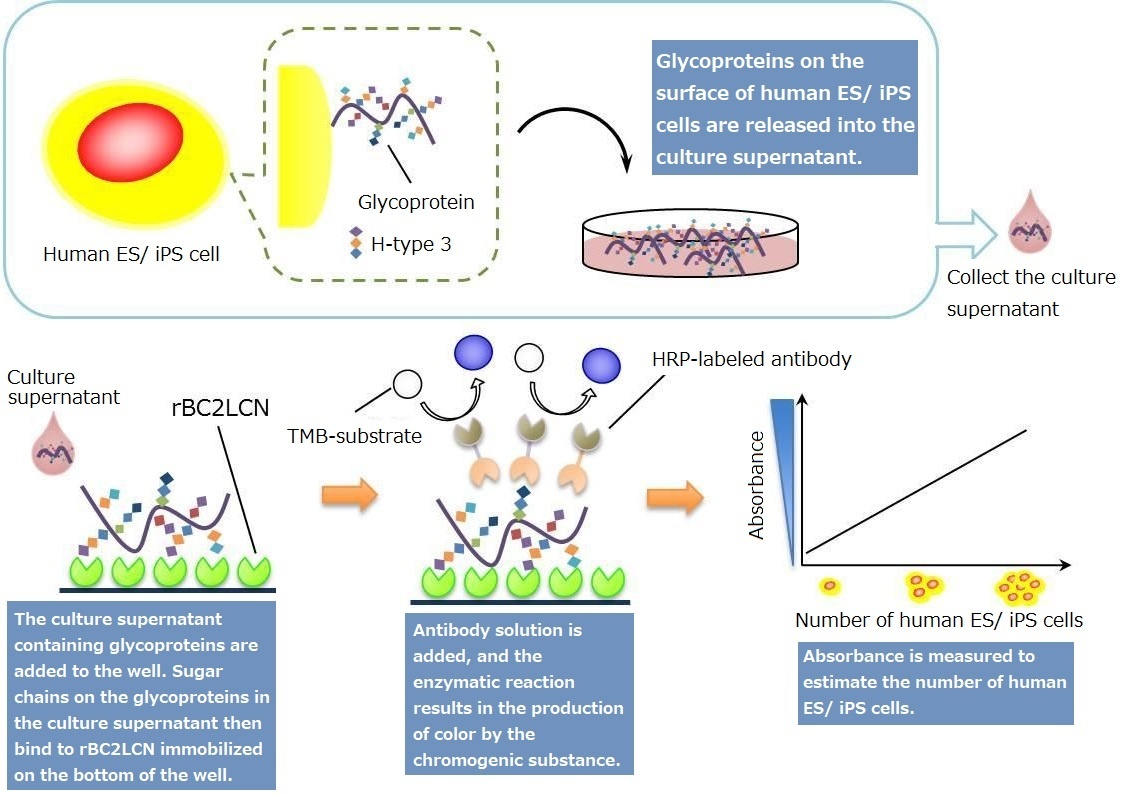
Human ES/ iPS Cell Monitoring Kit
rBC2LCN(AiLecS1) is a recombinant protein of the N-terminal domain of BC2L-C (derived from Burkholderia cenocepacia ) expressed in E.coli. It is highly specific to sugar chains on the surface of undifferentiated human ES/ iPS cells. It has high affinity to H-type3(Fucα1-2Galβ1-3GalNAc, which is a mucin-type O-glycan sugar chain on podocalyxin expressed on the surface of human ES/ iPS cells. It is therefore used as a marker for undifferentiated human ES/ iPS cells.
Glycoproteins recognized by rBC2LCN (H-type3-binding podocalyxin) are released from the surface of human ES/ iPS cells into the culture medium. This kit enables quantification of glycoproteins released by human ES/ iPS cells into the culture medium by sandwich assay using rBC2LCN and antibody for undifferentiation markers. Thus, it can be used to estimate the number of human ES and iPS cells.
Features
- Enables monitoring of the change in the number of undifferentiated cells by analyzing culture supernatant.
- As the culture supernatant is used for the assay, no cells are wasted for the analysis and cells can be maintained in the culture.
- Requires only a small amount of culture supernatant (50 μL).
- As the assay is based on ELISA, multiple samples can be processed in a simple manner.
Kit Contents
| Components | Size x Number |
|---|---|
| rBC2LCN immobilized plate | 96well x 1 |
| HRP-labeled antibody solution | 400μL x 1 |
| Negative control | 1mL x 1 |
| Positive control | 100μL x 1 |
| Dilution solution | 15mL x 1 |
| Wash solution (10x) | 100mL x 1 |
| TMB solution | 10mL x 1 |
| Stop solution | 10mL x 1 |
| Plate seal | 4 |
Product Description
Principles
Creating a Standard Curve
The kit does not include a standard that can be used to create a standard curve. Please follow the steps below to prepare a standard before creating a standard curve.
- Culture human ES/ iPS cells while maintaining their undifferentiated states. Collect the culture supernatant 18-24 hours after replacing the culture medium.
- Detach the cells from the culture dish and count the number of human ES/ iPS cells.
- Calculate the total number of human ES/ iPS cells in the collected culture supernatant. For example, if 5×106 cells were counted in 5 mL of culture medium, the total number of human ES/ iPS cells in the culture supernatant would be 1×106 cells/mL.
- Prepare dilution series of the culture supernatant for known cell concentrations using either an undifferentiation or differentiation medium to create a standard curve.
Applications for differentiation condition
- Sample the culture supernatant of human ES/ iPS cells while they are maintained in their undifferentiated states. Count the number of human ES/ iPS cells.
- Dilute the culture supernatant from the previous step (step 1) with a differentiation medium. Prepare dilution series and create a standard curve.
- Using the kit, measure the sample of the culture supernatant that contain human ES/ iPS cells.
- Estimate the number of residual human ES/ iPS cells under the differentiation condition.
Comparison among cell lines and culture media
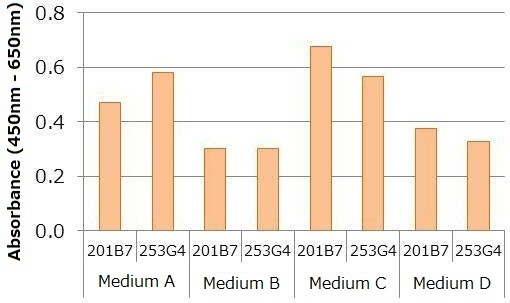
Human iPS cells line 201B7 and 253G4 were cultured under a undifferentiation condition (medium A, B, C, D), and culture supernatants were collected for each sample one day after replacing the culture medium. The number of cells were counted, and each sample was diluted to the final concentration of 2,000 cells/mL using respective undifferentiation medium. Absorbance was then measured using the kit. The data shown below indicates variance in the signal intensity for different cell lines and culture conditions (i.e. medium) even though the number of cells (cells/ mL) was kept consistent. This suggests that a standard curve should be created for each culture condition.
Measuring human iPS cells in culture supernatant
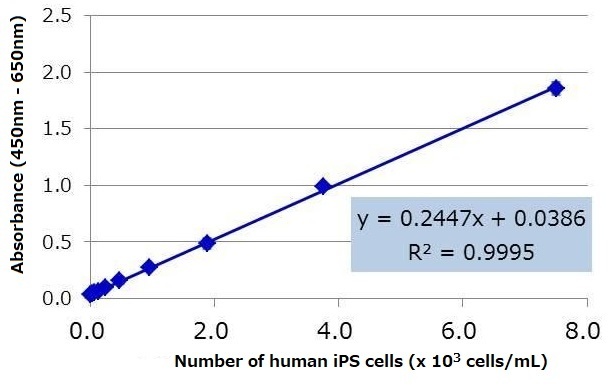
Human iPS cell line 201B7 was cultured in StemSure® hPSC medium Δ, and the culture supernatant was collected one day after replacing the medium. The number of cells were counted, and dilution series of the culture supernatant were prepared with StemSure® hPSC medium Δ. The diluted samples were analyzed using the kit, and the resulting regression equation had a high correlation coefficient. Based on the regression equation, the lower limit of detection※ for human iPS cells under this particular culture condition was calculated to be 108 cells / mL.
Spiking experiments using human iPS cell supernatants with supernatants of a differentiation medium
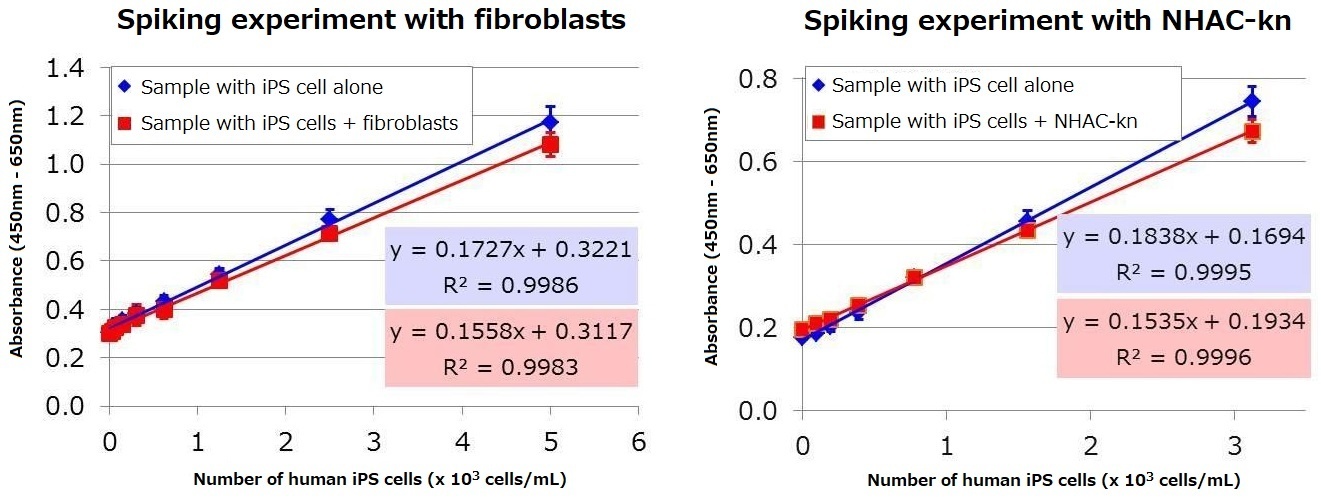
Mixed samples were analyzed to determine whether human iPS cell culture supernatant can be assayed when added to a culture supernatant containing either fibroblasts or human knee articular chondrocytes (NHAC-kn) serum. Human iPS cell culture supernatant was mixed with fibroblast culture supernatant or NHAC-Kn supernatant, and the proportion of human iPS cells were set to be between 0-20% (0-2×104 cells/mL: total cell number 1×105 cells/mL). The samples were then assayed using the kit. The result demonstrated that human iPS cell supernatant can be assayed even in the presence of serum and fibroblasts. Based on the regression equation obtained from the mixture of iPS cells with either fibroblast or NHAC-Kn, the lower limit of detection※ for human iPS cells under these particular culture conditions were calculated to be 170 and 96 cells / mL, respectively.
Note
- The relationship between the signal intensity and number of cells (cells/mL) can vary depending on the culture conditions (i.e. cell line, type of culture medium). A standard curve should be created for each cell line and differentiation/ undifferentiation conditions.
- The degree of undifferentiation cannot be determined based on the signal intensity under different culture conditions. Evaluations should be done under a consistent condition.
- The number of human ES/ iPS cells calculated from this kit does not necessarily correspond to the actual number of cells. Consider it as one of the indicators to monitor the state of undifferentiation and the progression into a differentiated state.
Q&A
Q1. Does podocalyxin remain in the culture supernatant?
A1. Podocalyxin is released into the culture supernatant. The signal does not decrease even after storing the culture supernatant for 8 days under 37°C. Thus, the sugar chain on podocalyxin to be assayed by this kit is stable. It is stable for up to 4 months if stored in a freezer; however, multiple thawing and freezing should be avoided.
Q2. Can podocalyxin be used as the standard?
A2. The kit detects sugar chains on podocalyxin. It would be challenging to create a standard that is suitable for the kit as proteins with sugar chains cannot be synthesized easily. Although it may be possible to create podocalyxin without sugar chains, it may not be suitable as the standard.
Q3. Is the size of podocalyxin released into the supernatant consistent?
A3. Polocalyxin is a 55 kDa protein with specific amino acid sequence. However, it exists as a large mucin-like molecule (> 240 kDa) with O-glycan sugar chain, N-glycan sugar chain, and glycosaminoglycans. It is a heterogenous glycoprotein that exists in multiple forms with varying degree and type of glycosylation. Thus, the molecular weight is not consistent. In electrophoresis, it appears as a smear with > 240 kDa in size.
Q4. Is podocalyxin be released even in a 3D culture?
A4. Yes- we have confirmed that podocalyxin is released into 3D culture.
Q5. What is the role of podocalyxin?
A5. Its role in human iPS cells is still unknown. However, studies suggest that it has a role in the maintenance of undifferentiated states, as well as in proliferation and differentiation of cells. In kidneys, it is a main glycoprotein expressed in glomerular podocytes and play a role in the formation and function of podocytes. Lack of podocalyxin in mice leads to kidney dysfunction and death.
Q6. When collecting the culture supernatant, should the medium be mixed?
A6. Lightly mix the medium by shaking the dish/ plate before collecting the culture supernatant.
Q7. What are the positive and negative controls for?
A7. Use the positive and negative controls to ensure that the components of the kit are functioning properly.
Reference
Tateno, H., Onuma, Y., Ito, Y., Hiemori, K., Aiki, Y., Shimizu, M., Higuchi, K., Fukuda, M., Warashina, M., Honda, S., Asashima, M. and Hirabayashi, J.: Sci. Rep., 4, 4069 (2014).
Product List
- Open All
- Close All
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



