[Chromatography Q & A] Methods to Remove Proteins in Biological Samples
When HPLC analyzing low-molecular substances in biological samples containing a large amount of proteins such as serum, deproteinization is necessary. As shown in Table 1, methods utilizing various principles are used. Here, the major ones will be introduced.
Table 1. Various deproteinization methods
| Principle | Method | Example |
|---|---|---|
| Insolubilization due to protein denaturation | Addition of acids | Perchloric acid, trichloroacetic acid, metaphosphoric acid |
| Addition of organic solvents | Acetone, acetonitrile, methanol, ethanol | |
| Heating / cooling | ||
| Physical removal | Ultrafiltration | Membrane filter (centrifugal filter device, etc.) |
| Dialysis | Dialysis tube | |
| Ultracentrifugation | ||
| Direct injection | Use of Restricted Access Media (RAM) | Internal-Surface Reverse-Phase (ISRP), hybrid packing agent, hydrophilic polymer packing agent |
Insolubilization Due to Protein Denaturation
This is a method to denature and precipitate proteins by breaking the higher-order structure of proteins. In many cases, methods which add acids or organic solvents are used. Although these methods are widely used, unexpected chemical reactions may occur, and it is necessary to confirm in advance that they do not affect the concentration of the target substance. Moreover, after protein precipitation, they require time and effort such as centrifugation and collection of supernatant.
Physical Removal
Ultrafiltration and dialysis are methods which use molecular size differences to separate proteins from the target low-molecular substance. The conditions are mild, and thus there is little concern about side reactions. Centrifugal products enable ultrafiltration by simple operation. A membrane with a molecular weight cutoff suitable for the purpose is selected, taking account of nonspecific adsorption of the target substance and the ability of the membrane to allow a fluid to permeate through. In addition, selection of membrane and centrifugation conditions are affected by the properties of the sample, its concentration, pH etc., and thus it is necessary to confirm them by a preliminary experiment. Various products are commercially available.
Use of Restricted Access Media (RAM)
In contrast to the previous 2 methods of injecting samples into HPLC after deproteinization, in this method, samples containing proteins are directly injected and deproteinized on line. Columns with RAM are used which have a function that high-molecular substances such as serum proteins are eluted by size exclusion without denaturation, and low-molecular substances such as drugs are retained by hydrophobic or electrostatic interaction. In many cases, a column switching method is used which deproteinizes using a column with RAM and analyzes the target component using another column. This method is unique in that the sample can be injected into the HPLC just by passing through a filter of about 0.2 μm. Here are examples of analyses of a drug in serum using Wakopak® Wakosil® GP-N6 (column packed with silica-based packing agent modified with a functional group having hydrophobic and hydrophilic phases).
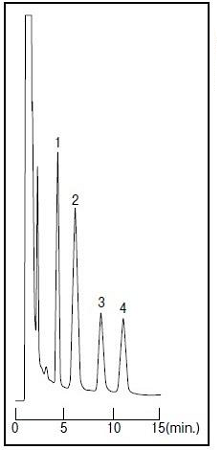
| Column Eluent Flow Rate Detection Sample |
: Wakopak® Wakosil GP-N6 (4.6 mm x 150 mm) : CH3OH/0.1 M Na2HPO4 (pH 6.0)=10/90 (v/v) : 1.0 mL/min. at 40℃ : UV 254 nm :1. Acetaminophen 5 μg/mL 2. Sulfamethoxazole 5 μg/mL 3. Ethenzamide 20 μg/mL 4. Salicylamide 25 μg/mL |
Figure 1. Direct Analysis of drugs in serum using Wakopak® Wakosil® GP-N6
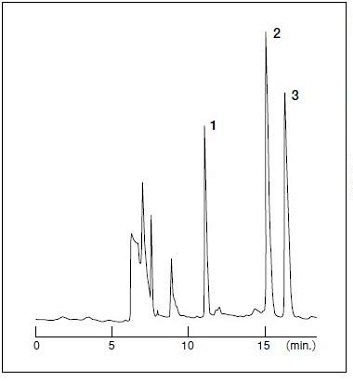
[Pretreatment conditions]
| Column Eluent Flow rate |
: Wakopak® Wakosil GP-N6 (4.6 mm x 50 mm) : CH3CN/0.2 M NaH2PO4, Na2HPO4 (pH 6.0)=2/98 (v/v) : 1.0 mL/min. at 30℃ |
[Analytical conditions]
| Column Eluent Flow rate Detection Column switching time Sample |
: Wakopak® Wakosil-Ⅱ 5C18 RS (4.6 mm x 150 mm) : CH3CN/0.1 M NaH2PO4, Na2HPO4 (pH 6.0)=30/70 (v/v) : 1.0 mL/min. at 30℃ : UV 254 nm 0.032 AUFS : 4.8-6 min. : Human serum 20 μL 1. Phenobarbital 20 μg/mL 2. Carbamazepine 5 μg/mL 3. Phenytoin 40 μg/mL |
[Drug recovery rate]
| Name of drug | Blood concentration (μg/mL) | Recovery rate (%) | CV (%) |
|---|---|---|---|
| 1. Phenobarbital | 20 | 99.3 | 1.06 |
| 2. Carbamazepine | 5 | 96.5 | 1.12 |
| 3. Phenytoin | 40 | 102.3 | 0.78 |
Precautions on handling
- Please use the mobile phase with the pH range of 2.5 to 7.5.
- Use the organic solvent concentration in the mobile phase at a level that does not denature the proteins (for acetonitrile, we recommend 20% or lower). Do not use solvents that denature the proteins.
- Pass the sample through a 0.2 μm filter.
- To prevent clogging of the column filter, it is recommended that a line filter of approximately 2 μm should be connected between the autosampler and column.
- Please handle the column in the same manner as the reversed-phase columns.
Figure 2. Analysis of drugs in serum using Wakopak® Wakosil® GP-N6
and Wakopak® Wakosil-Ⅱ 5C18 RS (column switching method)
The major deproteinization methods were shown above. The use of centrifugal ultrafiltration and RAM is an effective means, especially when processing multiple samples.




