What is the Detection Limit of Endotoxin in Water?
This article was written by Dr. Masakazu Tsuchiya, FUJIFILM Wako Pure Chemical Corporation, for Vol. 63, No. 4 (October 1995) of Wako Junyaku Jiho.
The content of this article is from the time of publication. It is not the latest information due to new knowledge and changes in regulatory rules after original publication.
The LAL test for the measurement of endotoxins was developed primarily to ensure the safety of pharmaceuticals. For example, the allowable endotoxin limit for water for injection is 0.25 EU/mL, but this concentration is quite high considering the current techniques which are available for the detection of endotoxins.
Samples that have a potentially high level of interference with the measurement must be diluted. Therefore, it is necessary to have the ability to measure very low concentrations of endotoxin. In addition to keeping the endotoxin level in a sample below the standard, continuous quantification of endotoxin is required in some settings such as quality management systems for water used in manufacturing.
Therefore, it is important to determine the detection limit for the quantification of endotoxin. This article focuses on ways to measure very low concentrations of endotoxin. For the purpose of this article, I will be using the unit EU/L instead of EU/mL to express the concentration of endotoxin.
Among the products available at FUJIFILM Wako Pure Chemical Corporation, the product "Limulus ES-II Single Test Wako" has the highest detection sensitivity. This product can be used with a Toxinometer® to measure endotoxin down to a level of 4 EU/L in 60 minutes. With longer measurement times, approximately 1 EU/L of endotoxin can be measured.
In one study we investigated various conditions for measurement and demonstrated that LAL sensitivity can be increased by increasing the volume of the sample 1). While the additional sample volume reduces the sensitivity by decreasing the concentration of the LAL reagent, it can also increase LAL sensitivity by increasing the ratio of endotoxin to LAL reagent in the reaction solution.
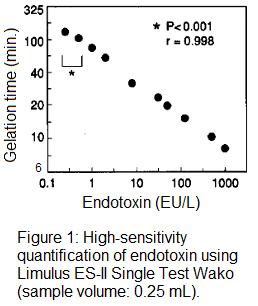
In this study we demonstrated that the detection sensitivity almost doubled when the amount of the sample was increased by 25-30%. This method doesn't apply to samples that have high levels of interference with the measurement, but it is applicable for samples such as water, which have no interference. In fact, we measured the level of endotoxin in water by adding 0.25 mL of the sample (instead of 0.2 mL which is normally used) to Limulus ES-II Single Test Wako, and detected 1 EU/L of endotoxin within 90 minutes (Figure 1).
Furthermore, the PyrosepTM method described in the previous article can be used to detect an even lower concentration of endotoxin with a Toxinometer® because it enables the endotoxin in a sample to be concentrated by the capillary column. Using 10 mL of a sample, we were able to concentrate the amount of endotoxin and detect 1 EU/L within 30 minutes. Moreover, when a 3 hour measurement was used we were able to detect endotoxin concentrations as low as 0.015 EU/L2).
Since it is difficult to detect such low concentrations of endotoxin using other methods, it is not possible to prove the accuracy of the measured value using the aforementioned methods. Future studies will have to confirm the reliability of endotoxin measurements at very low concentrations.
As discussed above, there have been a lot of technical advancements to improve the detection sensitivity of the LAL test. Newer methods enable endotoxin detection with a higher sensitivity in the range of less than one-thousandth of what can be detected by the conventional gel-clot method (0.03-0.25 EU/mL). For highly sensitive methods of detection, precautions must be taken to minimize contamination from the water, the instruments used, the environment, and other contaminants that may arise during the procedure. Extensive measures are required to prevent such contamination.
Various technical advancements for the LAL test have contributed to the recent development of high-sensitivity quantification methods. With further improvements in the instrumentation and techniques used, we aim to broaden the applicability of the LAL test.
References
- 1) Haruhisa Ijiri et al. Proceedings for the 20th Annual Meeting of the Society for antibacterial and Antifungal Agents. p.34 (1993).
- Masakazu Tsuchiya et al. Proceedings for the 22nd Annual Meeting of the Society for antibacterial and Antifungal Agents. p.121 (1995).




