What is the Purpose of an Endotoxin Recovery Test?
This article was written by Dr. Masakazu Tsuchiya, FUJIFILM Wako Pure Chemical Corporation, for Vol. 64, No.1 (January 1996) of Wako Junyaku Jiho.
The content of this article is from the time of publication. It is not the latest information due to new knowledge and changes in regulatory rules after original publication.
Inhibition and enhancement testing are required for validation of the endotoxin test. A recovery test is performed for inhibition and enhancement testing, whereby a sample containing a known amount of endotoxin is measured using the LAL test. The recovery rate is calculated as the ratio of the amount of endotoxin measured to the amount of endotoxin added.
This article will focus on the meaning of the recovery test.
What is the purpose of an endotoxin recovery test? The recovery test is used to validate the measurement of endotoxin activity in a sample. In other words, it is performed to determine if the interference of coexisting substances with the measurement is within an acceptable range.
In a recovery test for substances other than endotoxin, such as for sugars or inorganic compounds, the substances are quantified by their mass. The mass of a substance remains unchanged unless the substance is decomposed or transformed by the sample. In this case, the interference of coexisting substances with the measurement is equal to the effect of the sample on the reaction system.
The scenario is more complex for endotoxin because its activity cannot be simplified into an absolute amount such as mass. While the theoretical molecular weight of endotoxin is in the range of a few thousand to 20,000 Daltons, its actual molecular weight can range from a few hundreds of thousands to a few million Daltons due to the micelles that can form in aqueous solutions1).
Furthermore, the size of a micelle determines the biological activity of endotoxin, which includes its reactivity to the LAL test. Thus, the endotoxin test measures the activity of endotoxin rather than the absolute amount of endotoxin.
The activity of endotoxin may change in an inverse manner. Therefore, the effects of a sample on both the reaction system and endotoxin activity should be considered when determining the effects of interfering substances on the measurement (Figure 1).
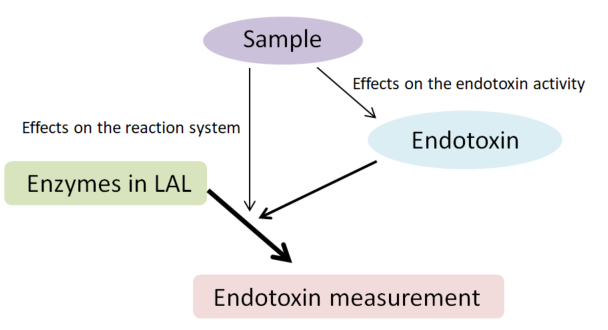
Figure 1: Effects of a sample in an endotoxin recovery test
In a typical endotoxin recovery test, the LAL test is performed after adding a known amount of endotoxin to a sample. This test simultaneously measures the effect of the sample on the reaction system and the effect of the sample on endotoxin activity. In most cases, the procedure for the measurement of endotoxin in a sample is determined based on this method.
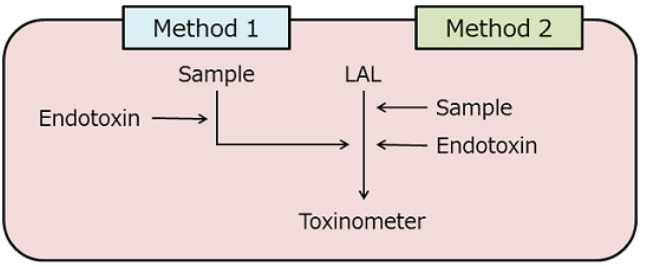
Figure 2: Conventional (method 1) and improved (method 2) methods for endotoxin recovery test.
There are some cases in which the measurement of endotoxin in a sample is not reliable. For example, as described in Episode 10 of this series, the presence of a small amount of ferric chloride reduces the activity of endotoxin 2). We performed a conventional endotoxin recovery test on a sample containing 40 µM of ferric chloride (Figure 2, Method 1) and obtained the recovery rate of 13.4%. This suggested that further dilutions were needed to measure endotoxin accurately.
We then modified the method of endotoxin addition and performed the test using Method 2 (Figure 2), resulting in 88.7% recovery. This finding indicates that while ferric chloride did not have much influence on the enzyme system of the LAL test, it did reduce the activity of endotoxin to less than 20%.
Another issue is that the effects of a sample on the activity of endotoxin vary with the type of endotoxin added to the sample. Most control standard endotoxins that are used as references and are commercially available contain additives. These additives are used to stabilize the activity of endotoxin.
In the previous example with ferric chloride, the addition of albumin to the endotoxin solution would suppress the inhibition of endotoxin activity. Therefore, the outcome of a recovery test may depend on the type of endotoxin solution used in the recovery test.
In conclusion, there are two questions that need to be addressed when performing an endotoxin recovery test: 1) Is it necessary to distinguish the effects of the sample? and 2) What type of endotoxin should be used?
References
- Makoto Niwa. Endotoxin: Its structure and activity. Edited by Yuzuru Honma, p.214. Ishiyaku Publishers, Inc. (1983).
- Masakazu Tsuchiya. Wako Junyaku Jiho, 61(1), 12(1993).




