Pyrogen Detection: Endotoxin, Peptidoglycan and β-D-Glucan
Endotoxin present on the cell wall of gram-negative bacteria is said to exhibit various biological activities such as exothermicity when entering the blood, and nowadays endotoxin tests are essential to be performed for those entering the blood by injections, medical instruments, etc.
In 1964, it was discovered that the endotoxin caused the aggregation and coagulation of the blood cell extract from limulus, and the lysate reagent was developed using this phenomenon.
There are two kinds of Limulus to be the raw material of lysate reagent, which are Limulus polyphemus inhabiting on the east coast of America and Tachypleus tridentatus inhabiting in the Southeast Asia. The lysate reagent prepared from the blood cell extract from Limulus polyphemus is called Limulus Amebocyte Lysate, commonly LAL.
On the other hand, the lysate reagent prepared from the blood cell extract from Tachypleus tridentatus is called Tachypleus Amebocyte Lysate, commonly TAL.
Product Line-up
What is bacterial endotoxin?
An endotoxin is a lipopolysaccharide (LPS) found in the cell wall of gram-negative bacteria. It is a typical pyrogen, which induces various biological reactions when even a small amount of pg (10-12 g) or ng (10-9 g) enters the bloodstream. Due to its heat resistance and stability, complete inactivation of endotoxin is not possible with autoclaving. Dry heat sterilization for at least 30 minutes at a temperature of 250 °C or more is required complete inactiocaiton,. It exists in the environment (e.g. water, air) inhabited by gram-negative bacteria, and bacterial endotoxins (LPS) remain even after the bacteria die.
Figure 1 shows the LPS structure schematic, which illustrates lipid A as the component responsible for the bioactivity. The molecular weight of this portion is approx. 2,000. The entire molecular weight, including the sugar chain moiety, is usually approx. 5,000 to 8,000. However, since an LPS consists of a hydrophilic region (sugar chain) and hydrophobic region (lipid A), it associates in an aqueous solution to form a micellar structure with apparent molecular weight of hundreds of thousands to several millions. A change in the micellar structure reportedly influences the strength of bioactivity.
Figure 2 illustrates the structures of Salmonella-type and E. coli-type lipid A, which indicate that the basic structure of the lipid A is incommon, regardless of the strain variation.
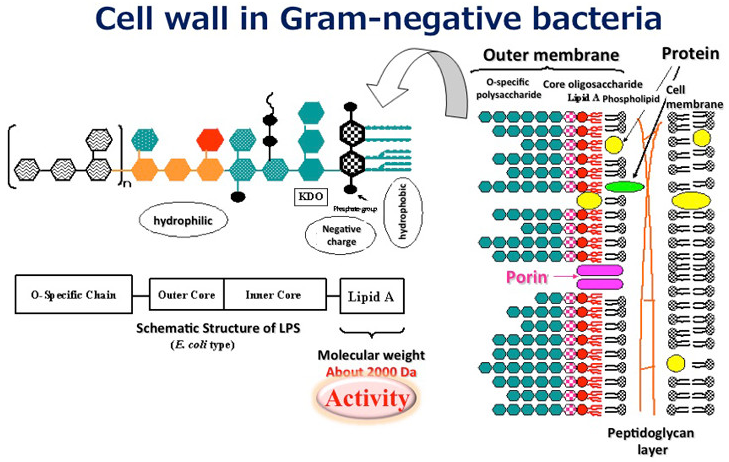
Figure 1: Lipopolysaccharide Structure Schematic Diagram
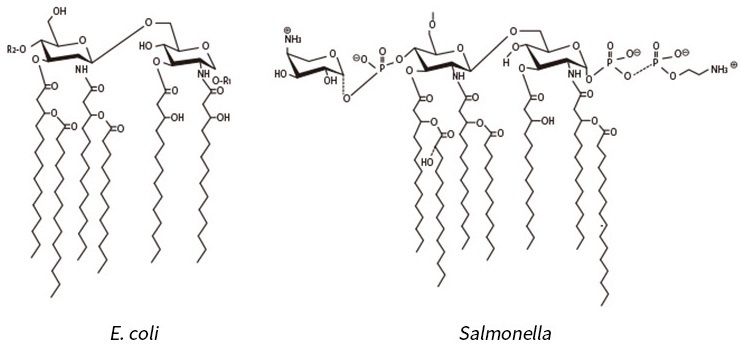
Figure 2: Lipid A Structure (E. coli and Salmonella types)
Reaction Mechanism
A lysate reagent prepared from the amebocytes of horseshoe crab (Limulus polyphemus) is used to detect bacterial endotoxins. As shown in Figure 1, the cascade reactions start by the presence of an endotoxin, whereby Factor C, a serine protease precursor, is initially activated. There follows the subsequential activation of Factor B, also a serine protease precursor and a proclotting enzyme, which hydrolyzes coagulogen into coagulin, forming an insoluble gel.
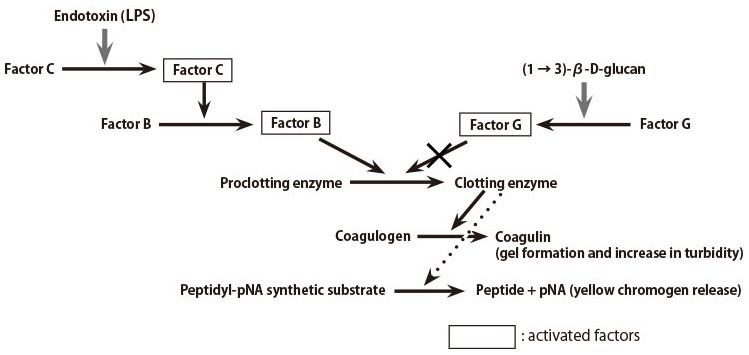
Figure 1: LAL Reagent Reaction Cascade Mechanism
Ordinary LAL reagents react not only with the endotoxin but also (1 → 3)-β-D-glucan (a fungal cell wall component), since the Factor G pathway can be activated in the reagents. To eliminate this (1 → 3)-β-D-glucan activation, various endotoxin-specific reagents are being developed in industry by removing Factor G or inhibiting its activation.
Various LAL reagents are commercially available, as well as measuring systems based on the Figure 1 reaction mechanism. It is essential to select the most appropriate product depending on the required accuracy, test frequencies, number of samples and other relevant factors.
Bacterial Endotoxins Test (BET), namely the gel-clot technique, turbidimetric technique and chromogenic technique, is harmonized with the Japan Pharmacopoeia , the European Pharmacopoeia and the U.S. Pharmacopeia.
Contribution to Measurement Technology - Acquisition of FDA approval
Endotoxin is a potent toxin contained in cell wall components of Gram-negative bacteria. Since it brings about the fever or lethal shock when entering the blood of a person, it is necessary to confirm that medicines are not contaminated with endotoxin. We have strived to evolve the endotoxin measurement technology by manufacturing lysate reagents and developing the measurement system"Toxinometer", and supported medicines from behind for over 30 years.
From the import and sales of lysate reagents to the development of endotoxin measurement system

Our US Plant (Location: Richmond, Virginia)
Initially, the rabbit pyrogen test was used for the endotoxin test method. After injecting the sample into the rabbit, the amount of endotoxin contamination was measured from the increasing degree of its body temperature. However, with this test method, there were drawbacks such as taking time to get results, being unable to maintain the reliable results because of using animals.
In 1956 in the United States, F.B.Bang discovered a phenomenon that the blood of horseshoe crabs solidified in a gel state with endotoxin, and then the endotoxin measurement reagent (Limulus reagent, renamed as lysate reagent later) prepared from the blood extract of horseshoe crabs and "Gel-clot technique" using this were developed. Gel-clot technique were listed in the US Pharmacopoeia in 1980, but we also began importing and selling the reagent prior to this. However, in the gel-clot technique, problems remained such as being the semi-quantitative method and the judgment accuracy depending on the proficiency of the measurer.
We challenged to solve these problems, then in 1985, developed the measurement method "Turbidimetric time analysis method" based on the different principle from the conventional one and released its measurement system "Toxinometer" for the first time in the world. At the same time, in the United States, ACC company (Associates of Cape Cod, .INC) developed a system using the same measurement principle. In response to this situation, the turbidimetric time analysis method was listed in the US FDA Guideline in 1987.
Currently, Toxinometers corresponding to the turbidimetric, chromogenic and gel-clot technique are provided along with numerous reagents. Meanwhile, also offering "MPR Endotoxin Measurement System" corresponding to the chromogenic and turbidimetric techniques using plate readers, we are contributing to the safety of pharmaceutical production and quality control of pharmaceuticals as a pioneer of endotoxin measurement.
Making efforts to disseminate endotoxin measurement technology
Currently, three endotoxin test methods, gel-clot technique, turbidimetric technique and chromogenic technique are listed in the pharmacopoeia. There are various test methods such as β-glucan measurement and peptidoglycan measurement (SLP-HS Single Reagent Set Ⅱ) in addition to the pharmacopoeia, and there are precautions in conducting each test method.
For example, in the endotoxin test, the careful attention is required for containers to be used, test water, etc. In the case based on the Pharmacopoeia the implementation of the preliminary and quantitative tests are stipulated. The method of adding the endotoxin and how to find the Maximum Valid Dilution may be difficult for the beginners. On the other hand, the samples have many variations. The measurement method must be appropriately adjusted according to the properties of samples hardly soluble/insoluble in water, or the subjects such as animal blood and culture medium, and in some cases, the ingenuities not described in the manual are actually required.
Establishment of the FDA approved plant and development of the world's only specific reagent for endotoxin
The world's first lysate reagent was developed in the United States, and we initially handled only imported items from the United States. As the market expanded and the needs of reagents increased, we established a plant in the United States. First of all, we bought Hemakem Inc. in St. Louis in 2003 and made it a foothold for entering the United States. After that, we built a new plant in Richmond, received FDA approval, and began shipping reagents. Incidentally, the endotoxin specific reagents with FDA approval are only our products worldwide.
Also, there is a convenient single type reagent that is lyophilized in a test tube beforehand and can be measured simply by putting the sample. Even in this category, the company having the endotoxin specific reagents with FDA approval is only us in the world.
As described above, our company, which has been involved in endotoxin reagents for more than 30 years and making efforts on the improvement of measurement methods and the development of "Toxinometer", has tried hard to develop the pharmaceutical industry and others, as one of pioneers of endotoxin measurement.
Product Line-up
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.