Endotoxin Measurement Systems
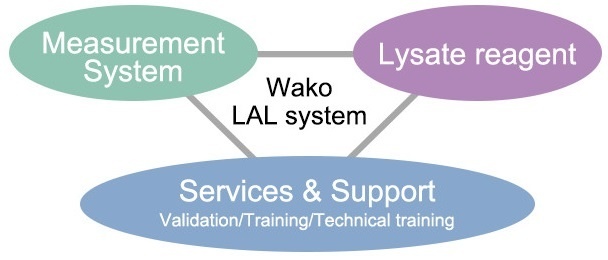
There are three methods of endotoxin test method, gel-clot technique, turbidimetric technique and chromogenic technique listed in the Pharmacopoeia, and the features, methods and precautions at the time of implementation are different with each test method.
On the other hand, their measurement targets are also varied, and it is necessary to establish the measurement conditions appropriately according to the characteristics and management standards. We prepare various manuals and standard books as the guidance for those engaged in the same test, but devising not described in the manuals will be also required during the actual testing work.
Therefore, we regularly hold the training courses and also carry out the in-house seminars and individual training etc, according to your request, and support that you can understand the test methods deeply.
Product Line-up
Bacterial Endotoxin Test and FUJIFILM Wako
We contributed to the establishment of the bacterial endotoxin test method etc. from the dawn of the test and have supported the development and dissemination of bacterial endotoxin test as well as the development of measurement system etc. meeting the needs of present age.
| Year | Bacterial Endotoxin test | Our LAL business |
|---|---|---|
| 1950-1956 | Bang conducted the blood clotting study of horseshoe crabs. | |
| 1964 | Levin & Bang discovered the endotoxin to clot the blood corpuscles of horseshoe crabs. *1 | |
| 1070 | Cooper, Levin, Wagner and others announced the possibility that LAL could be an alternative method to the rabbit pyrogen test (pyrogen test using rabbits). *2 | |
| Release of gel-clot technique reagents in Japan. | ||
| 1980 | Gel-clot technique were listed in the USP (US Pharmacopoeia) as Bacterial Endotoxins Test (BET). | |
| 1985 | Release of Toxinometer | |
| 1987 | FDA issued Validation Guidelines for Limulus Test. | |
| 1988 | Bacterial endotoxin test method (gel-clot technique) was listed in the Japanese Pharmacopoeia. (supplement 1 of the 11th edition) |
|
| 1993 | The international harmonization work on bacterial endotoxin test method was started. | |
| 1994 | Release of Limulus ES-II | |
| 1996 | In addition to the gel-clot technique, the turbidimetric technique and the chromogenic technique were listed in the Japanese Pharmacopoeia (the 13th edition). | The first Bacterial Endotoxin Test Method Seminar was held. Release of Limulus Color KY. |
| 1999 | Each country's pharmacopoeia signed the harmonization agreement in BET international harmonization plan. | |
| 2000 | LAL business was started at Wako Chemicals USA Inc. | |
| 2010 | Abolition of FDA Guideline for Limulus test. (Unified to USP BET) |
Release of Toxinometer ET-6000 |
| 2011 | FDA Guidance "Pyrogen and Endotoxins Testing: Q & A" was issued. | Wako Chemicals USA Inc.,: Richmond plant acquired FDA approval. |
| 2012 | International harmonization agreement of bacterial endotoxin test method. | Release of PYROSTAR ES-F |
| 2013 | BET International harmonization agreement Japan: March 21, 2013, Europe: May 2013, The United States: enforced on October 15, 2013. |
|
| 2015 | Release of Limulus ES-Ⅱplus CS |
References
*1: Levin,J. and Bang,F.B. Bull. Johns Hopkins Hosp.,115,265 (1964)
*2: Cooper JF, Levin J, Wagner HN. J Nucl Med 11,310 (1970)
Product Line-up
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.