Additives for LIB [WEA series]
LIB has a good potential for higher energy density and higher voltage than conventional batteries. In order to maintain the stable performance of the rechargeable battery, a technique for controlling the interface between the electrode and the electrolytic solution is required. We have developed additives that enable stable discharge and charge cycles. By supplementing a catalytic amount of an additive to the electrolytic solution, a coated layer is formed at the interface between the anode and the electrolytic solution.
Please visit another site for specialty chemicals, click HERE.
Features
- Reductive decomposition takes place to form a stable layer at the interface between the electrode and the electrolyte.
- he formed coating suppresses the decomposition of the electrolytic solution and enables stable high speed charge and discharge cycles.
Introduction
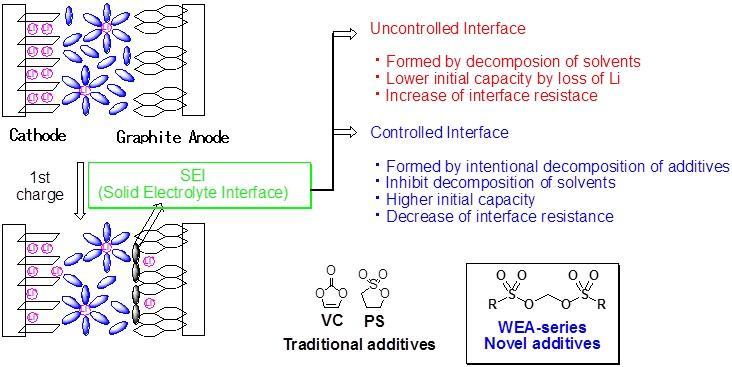
A coated layer called SEI, solid electrolyte interface, is formed when the LIB is charged for the first time. This layer suppresses the decomposition of the electrolyte and contributes to stable charge and discharge cycles.
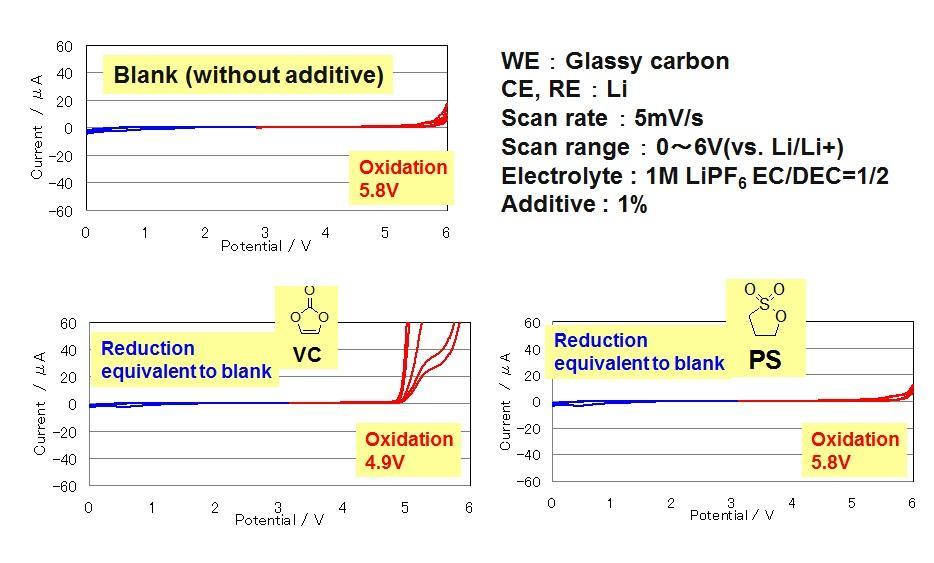
In general, vinylene carbonate or propane sultone is added as a supplement. A layer is formed along the controlled SEI by decomposition of the additive prior to electrolyte decomposition.
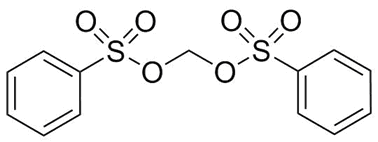
Methylene bissulfonates developed by FUJIFILM Wako Pure Chemical show lower LUMO calculated from computational chemistry, easily decompose by reduction and give good battery performance.
Applications
Cyclic Voltamograms of Electrolytes with Traditional Additives
Cyclic Voltamograms of Electrolytes with Novel Additives (WEA)
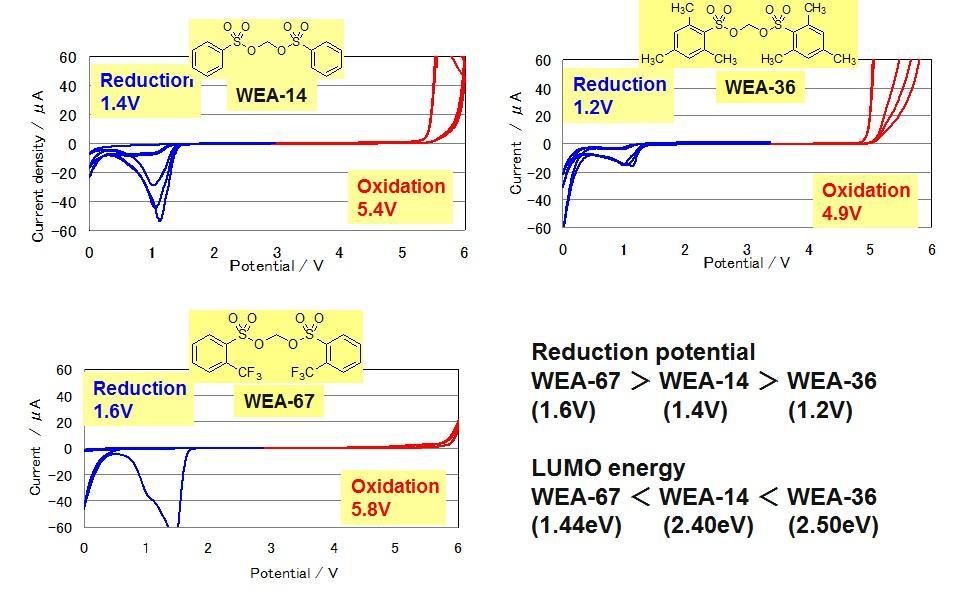
The WEA series shows a reductive decomposition peak clearly. The decomposition potential is the highest in WEA-67 having an electron-withdrawing group and WEA-67 is easily decomposed. On the other hand, WEA-36 having an electron-donating group shows the lowest potential. These results show the same trend as the LUMO energy calculated by computational chemistry.
Charge-Discharge Test
Conditions
Coin cell (CR2032)
| Cathode | LiCoO2 / AB / PVDF=85 / 7 / 8 (1.5mAh/cm2) |
| Anode | Natural graphite / AB / PVDF =86 / 6 / 8 (1.6mAh/cm2) |
| Electrolyte | 1M LiPF6 EC/DEC=1/2 |
| Additive | WEA (0.1%) |
| Temperature | 25°C |
| Voltage | 3.0-4.2 |
| Charge | CC-CV |
| Discharge | CC |
| Cycle test | 0.2C / 3cycle → 1.0C / 42cycle (total 45 cycles) |
| Rate test | 0.2C, 0.5C, 1C, 2C, 5C (after 45 cycles) |
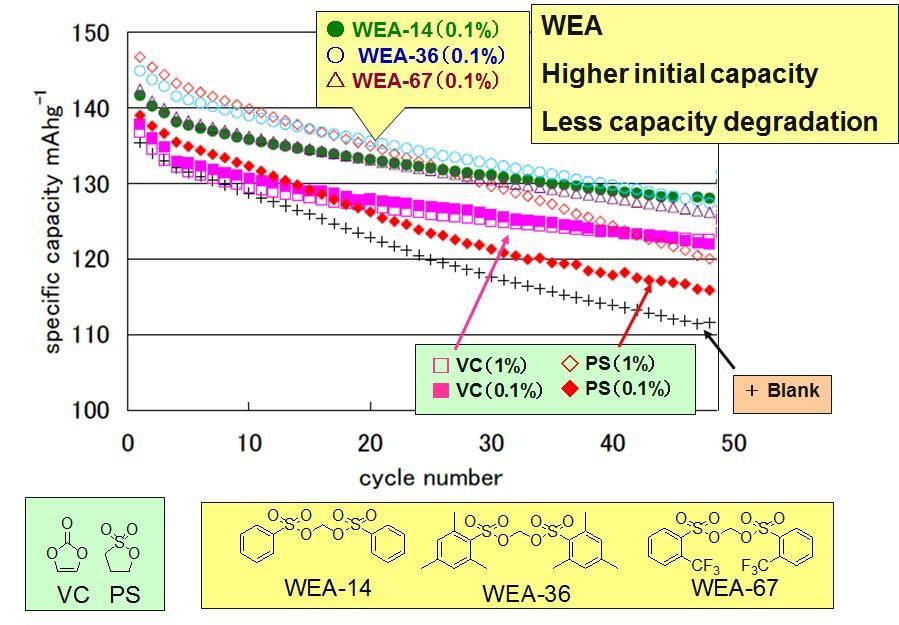
The addition of WEA increased the initial specific capacity. Also, the attenuation of cycle capacity can be improved.
dQdV Plot (1st Charge)
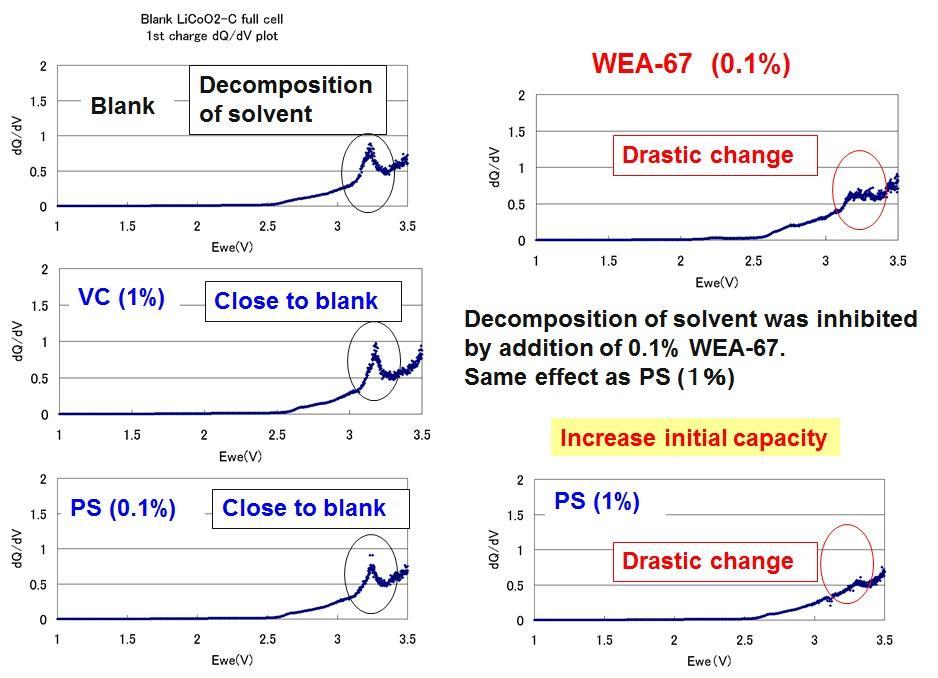
In order to confirm the improvement of the initial capacity, a dQdV plot is prepared. As a result, in the system to which WEA was added, no peak derived from decomposition of the solvent was observed. Th, it shows that WEA is a good battery additive.
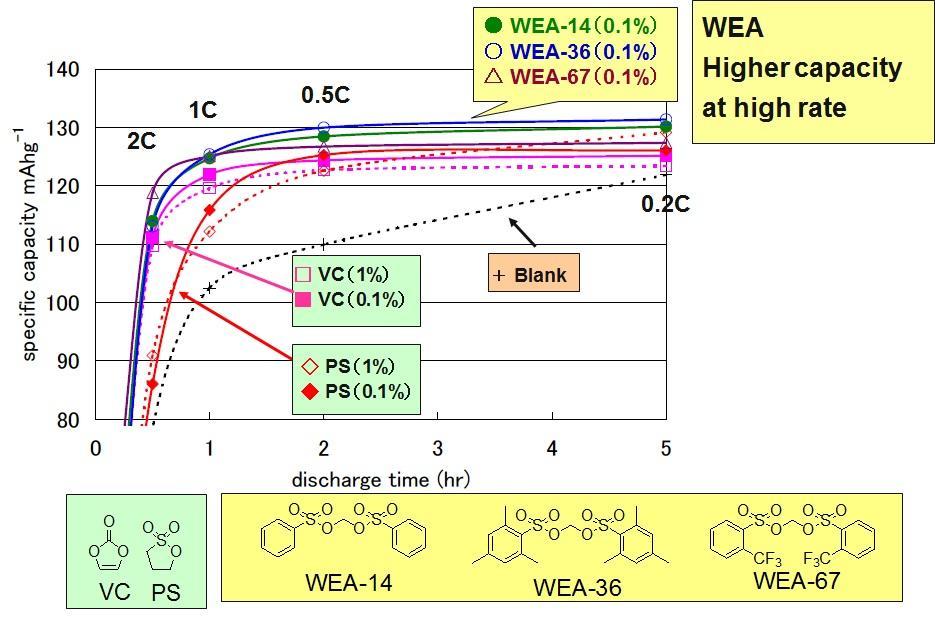
Rate Test (After 45 Cycles)
In the system to which WEA was added, the capacity was maintained and the performance was also improved.
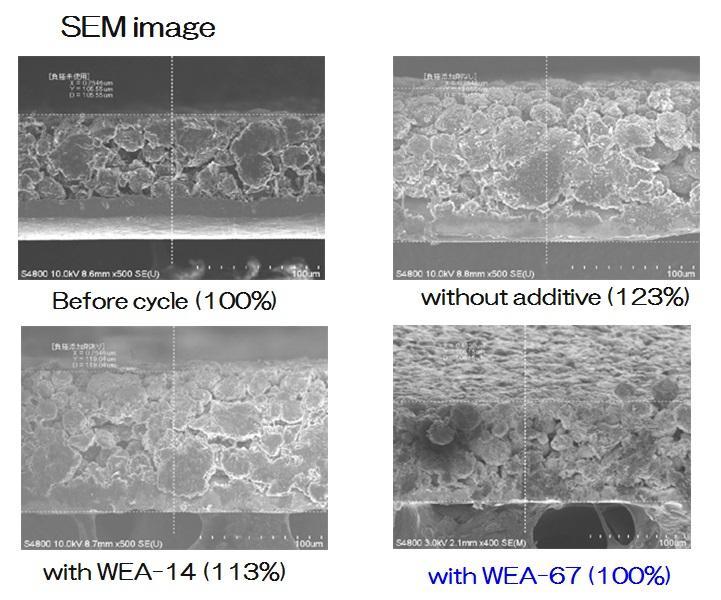
Comparison
Comparison of Expansion Rate (Graphite)
Product List
- Open All
- Close All
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.
The prices are list prices in Japan.Please contact your local distributor for your retail price in your region.



