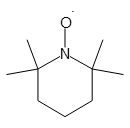
2,2,6,6-Tetramethyl-1-piperidinyloxy, Radical
- for Organic Synthesis
- Specification Assay :
- 98.0+% (Capillary GC)
- Manufacturer :
- FUJIFILM Wako Pure Chemical Corporation
- Storage Condition :
- Keep at 2-10 degrees C.
- CAS RN® :
- 2564-83-2
- Molecular Formula :
- C9H18NO
- Molecular Weight :
- 156.25
- GHS :
-
- Structural Formula
- Label
- Packing
- SDS
|
Comparison
|
Product Number
|
Package Size
|
Price
|
Availability
|
Certificate of Analysis
|
Purchase |
|---|---|---|---|---|---|---|
|
|
|
5G
|
|
In stock in Japan |
||
|
|
|
25G
|
|
In stock in Japan |
||
|
|
|
100G
|
|
In stock in Japan |
※Check availability in the US with the distributor.
Document
Overview
This product is a stable nitroxyl radical. In TEMPO-mediated oxidation, catalytic amount of TEMPO is used with reoxidant such as hypochlorite or hypervalent iodine. In general, when primary alcohol and secondary alcohol coexist, primary alcohol is oxidized in a functional group-selective manner into aldehyde. Also, reaction condition can be optimized to obtain carboxylic acid from primary alcohols.
Mechanism
In catalytic cycle, TEMPO is oxidized by the co-oxidant to generate oxoammonium cation which is actual oxidant. During the conversion of the alcohol to the corresponding aldehyde, oxoammonium cation is reduced to a hydroxylamine. The hydroxyl amine is reoxidized to oxoammonium cation by co-oxidant, thus completing the catalytic cycle.
Reaction
References
- Jauch, J. : Angew. Chem., Int. Ed., 39, 2764 (2000).
- De Mico, A., Margarita, R., Parlanti, L., Vescovi, A., Piancatelli, G. : J. Org. Chem., 62, 6974 (1997).
- Einhorn, J., Einhorn, C., Ratajczak, F., Pierre, J.-L. : J. Org. Chem., 61, 7452 (1996).
Overview / Applications
| Outline | This product is for research use only. Do not administer it to human.
|
|---|---|
| Precautions for Use | Packed on argon gas |
Property
| Appearance | Yellowish red - brown, crystals - crystalline powder or mass |
|---|
Manufacturer Information
Alias
- TEMPO
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.