Sodium Dihydrogen Phosphate Dihydrate [CertiPro:JPE,USP,Ph.Eur.,Endotoxin test]
- Japanese Pharmaceutical Excipients
- Specification Assay :
- 98.0+% (as NaH2PO4)(after drying)(Titration)(JPE)
98.0 - 103.0% (as NaH2PO4)(Titration)(USP-NF)
98.0 - 100.5% (as NaH2PO4)(Titration)(Ph.Eur.)
- Manufacturer :
- FUJIFILM Wako Pure Chemical Corporation
- Storage Condition :
- Keep at RT.
- CAS RN® :
- 13472-35-0
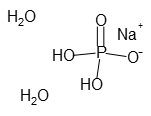
- Molecular Formula :
- NaH2PO4・2H2O
- Molecular Weight :
- 156.01
- Structural Formula
- Label
- Packing
- SDS
|
Comparison
|
Product Number
|
Package Size
|
Price
|
Availability
|
Certificate of Analysis
|
Purchase |
|---|---|---|---|---|---|---|
|
|
|
10kg
|
|
In stock in Japan |
||
|
|
|
500G
|
|
In stock in Japan |
※Check availability in the US with the distributor.
Document
Application
Overview / Applications
| Outline | It is compliant with added EP and USP tests to raw material supplements (grade) for pharmaceutical manufacturing. We have conducted endotoxin test and standardized it. |
|---|
Property
| Appearance | Colorless or white, crystals or crystalline powder(JPE) |
|---|---|
| Solubility | It is easily soluble in water and hardly soluble in ethanol (95).(JPE) very soluble in water, very slightly soluble in ethanol (96 per cent).(Ph.Eur) |
| pH | pH : 4.1 - 4.7 (JPE) pH : 4.1 - 4.5 (USP-NF) pH : 4.2 - 4.5 (Ph.Eur.) |
| Purity | Bacterial endotoxins : <2.0EU/g |
Manufacturer Information
Alias
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.