3-Methyl-3-buten-1-yl Methacrylate
- for Organic Synthesis
- Specification Assay :
- 98.0+% (capillary GC)
- Manufacturer :
- FUJIFILM Wako Pure Chemical Corporation
- Storage Condition :
- Keep at RT.
- CAS RN® :
- 156291-88-2
- Molecular Formula :
- C9H14O2
- Molecular Weight :
- 154.21
- GHS :
-
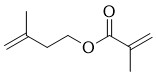
- Structural Formula
- Label
- Packing
- SDS
|
Comparison
|
Product Number
|
Package Size
|
Price
|
Availability
|
Certificate of Analysis
|
Purchase |
|---|---|---|---|---|---|---|
|
|
|
100mL
|
|
In stock in Japan |
||
|
|
|
500mL
|
|
In stock in Japan |
※Check availability in the US with the distributor.
Document
Product Summary
3-Methyl-3-buten-1-yl methacrylate (IPEMA) is a cross-linking agent with two different radical-reactive groups, which reduces the inhibition of curing by oxygen and accelerates curing under air. In addition, IPEMA acts as a reactive diluent to add hardness and flexibility to the cured film and can impart flame retardancy through the gradual addition of phosphate esters.
Physical Properties and Features
Physical properties
| Boiling point (℃) | Melting Point (℃) | Viscosity (25℃) (mPa・s) |
Refraction Index (d line, 25℃) |
|---|---|---|---|
| 180 | <-90 | 1.06 | 1.44 |
Features
- A cross-linking agent that has two olefins with different reactivities.
- In UV curing under air, it suppresses reaction inhibition by oxygen and accelerates curing.
- Useful as a reactive diluent that imparts hardness and flexibility to cured films.
Applications
Accelerated UV curing
Figure 1 shows the time course of UV curing using IPEMA as additives. Pentaerythritol triacrylate was used as a monomer and 1-hydroxycyclohexyl phenyl ketone was used as an initiator, and the change in the conversion of olefin was tracked using real-time IR measurements. While the curing proceeded with the conversion of 74.5% within 30 seconds under oxygen-blocked conditions (example 1), the conversion in the air remained at 4.3% due to the influence of oxygen (example 6). On the other hand, when IPEMA was added, the conversion was 44.5%, even in the air, and the conversion improved by more than 10 times (example 3). In addition, looking at the change in the conversion over time, it is also a significant feature that the addition of IPEMA causes the reaction rate to rise sharply immediately after UV irradiation, as in the case of the oxygen-blocking condition. Although the mechanism by which IPEMA promotes UV curing has not been clarified, it is presumed that having isoprenyl and methacryloyl groups in the same molecule contributes to the promotion effect, since the addition of isoprenol or methyl methacrylate has no effect in promoting UV curing.
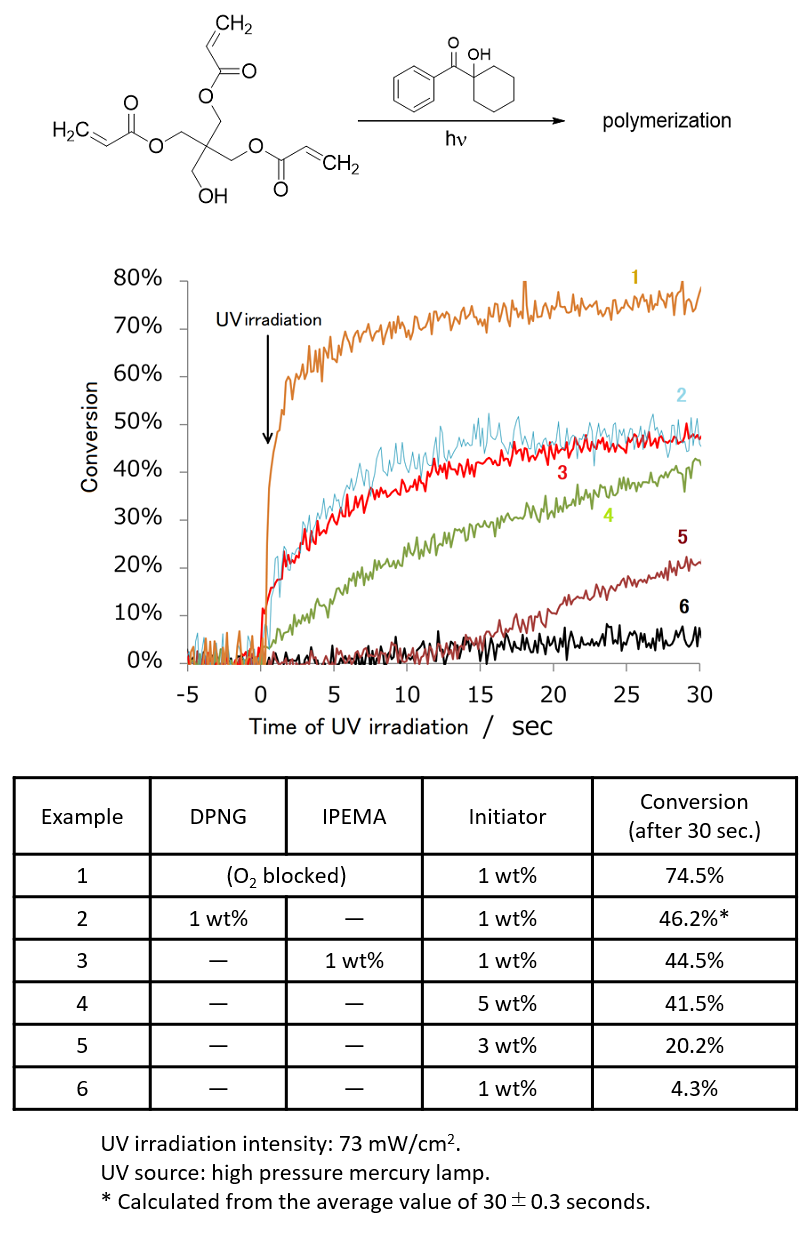 Figure 1. Change over time of olefin conversion by UV irradiation
Figure 1. Change over time of olefin conversion by UV irradiation
Reactivity of olefin in IPEMA
IPEMA is a bifunctional monomer with two different radically reactive groups, a methacryloyl group and an isoprenyl group. The Q-e values of their reactive groups were estimated from model compounds and are shown in Table 1. The methacryloyl group has a high Q-value (0.78) and a positive e-value (0.40), indicating that it is highly reactive, while the isoprenyl group has a low Q-value (0.034) and a negative e-value (-1.51), indicating that it is considerably less reactive than the methacryloyl group.
Therefore, IPEMA exhibits unique behavior when copolymerized with acryloyl groups (Figure 2). Methacryloyl groups easily polymerize with acryloyl groups and are incorporated into the polymer, whereas isoprenyl groups remain in the polymer until the middle stage of polymerization due to their low reactivity with acryloyl groups and low incorporation into the polymer. If the polymerization is continued, the isoprenyl group will eventually polymerize and participate in cross-linking (Figure 2, Reaction 1), but it is also possible to functionalize the remaining isoprenyl group (Figure 2, Reaction 2). Depending on the reaction pattern of these two isoprenyl groups, IPEMA can impart various functions to the resin.
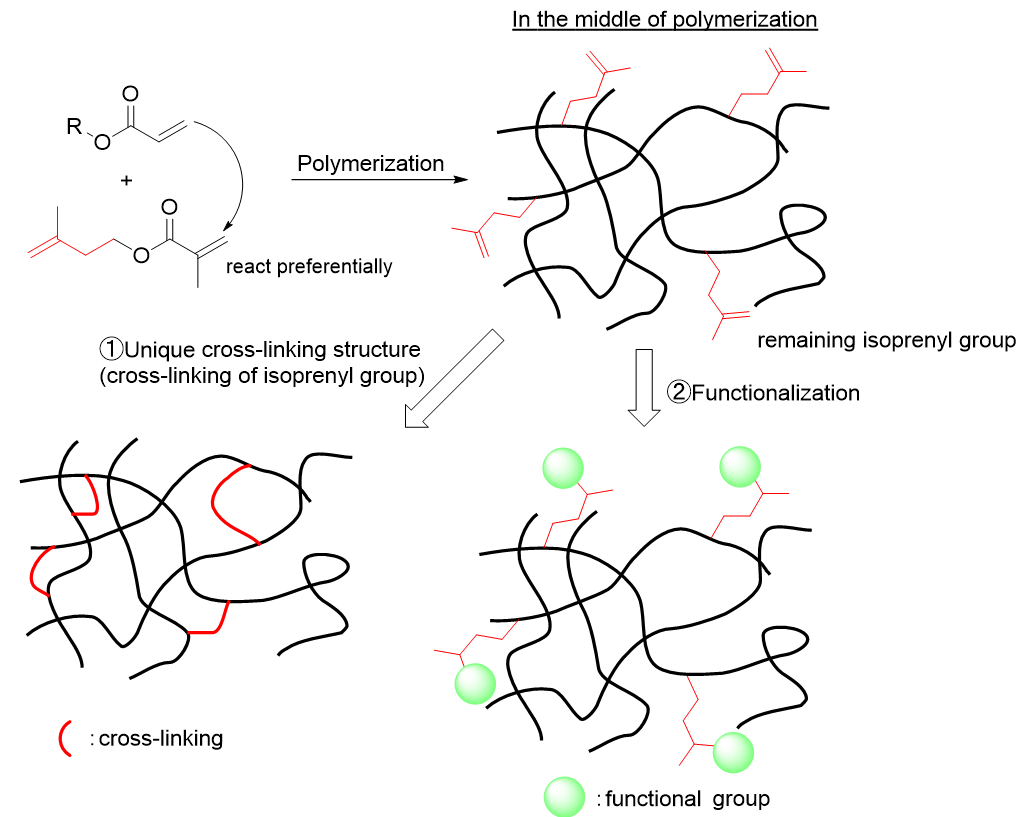 Figure 2. Unusual reaction behavior of IPEMA
Figure 2. Unusual reaction behavior of IPEMA
Addition of hardness and flexibility to cured film
As an example of the application of IPEMA's unique cross-linking structure, which is one of the reaction behaviors described earlier, we will introduce a reactive diluent used in the preparation of cured films. Reactive diluents are used to reduce viscosity and improve handling, but they can deteriorate curing properties and degrade the physical properties of the cured film.
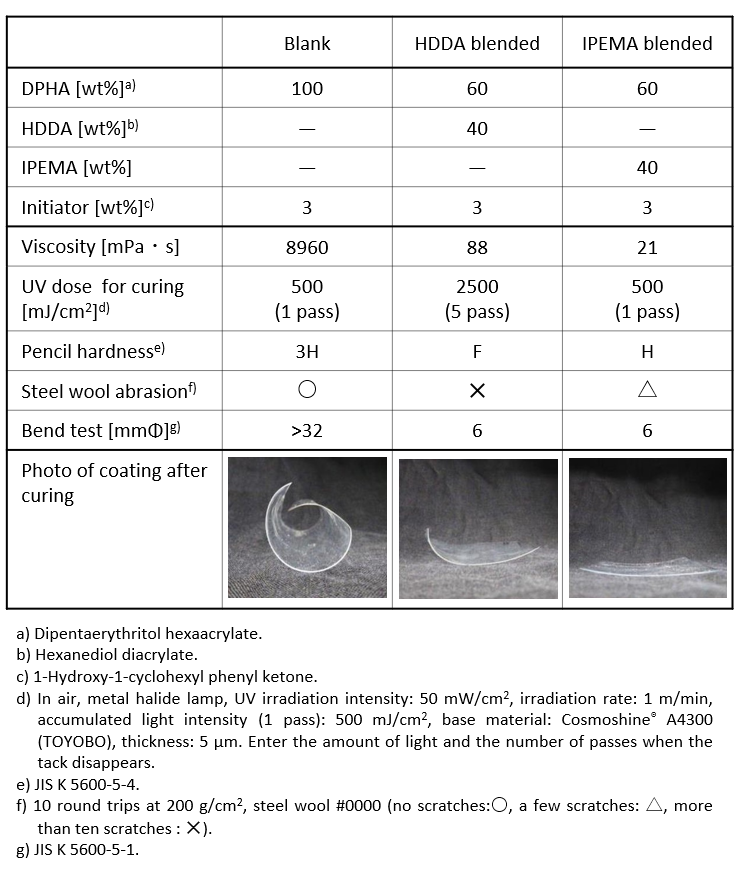
Table 2 shows the physical properties of the cured film when IPEMA is blended with polyfunctional acrylate as a reactive diluent. Compared to the bifunctional acrylate monomer HDDA, a common reactive diluent, IPEMA requires less light for curing while reducing viscosity, indicating that it is less susceptible to oxygen inhibition. In addition, the resulting cured film exhibits unique physical properties, including excellent surface hardness and abrasion resistance, as well as high flexural resistance of 6 mmΦ in the flexural resistance test. It can be said that the cured film containing IPEMA is an interesting material in terms of achieving both hardness and flexibility, which are inherently in a trade-off relationship. In addition, as shown in the photograph, the cured film containing IPEMA exhibits less warpage of the base material and curling is suppressed compared to the cured film containing HDDA.
These unique properties are thought to be derived from the molecular structure of IPEMA, which has a highly reactive methacryloyl group and an isoprenyl group incorporated at the end of polymerization in the same molecule. The different polymerization timing of the functional groups is interpreted as the progression of cross-linking with relaxed stress during curing and the formation of a unique cross-linked structure, leading to the unique functionality of IPEMA.
Table 2. Changes in coating properties with reactive diluents
Addition of flame retardancy to resin
As an example of the application of functionalized polymerization, another IPEMA reaction behavior, we will introduce the use of phosphoric acid esters to impart flame retardancy to resins. In recent years, the demand for non-halogen flame retardants has increased due to stricter regulations on flame retardants, and the development of non-halogen flame retardants, including phosphorus compounds, has been actively pursued. However, low-molecular-weight flame retardants have problems such as drip (melting dripping) and bleed-out of flame retardants, which can cause fire spread and spread when combustion occurs. IPEMA solves the problems of drip and bleed-out seen with low-molecular-weight flame retardants and makes it possible to easily achieve the nonflammability of resins.
Combustion of a general process acrylic plate using methyl methacrylate (MMA) as the main agent and a low-molecular-weight phosphate ester as a flame retardant, and an acrylic plate made by a functional polymerization process with the addition of IPEMA and phosphite is shown in Figure 3. While the general process drips during combustion due to the plasticizing effect of the low-molecular-weight phosphate ester, the functional group polymerization process suppresses dripping and achieves non-halogen non-flammability. It was shown that the introduction of phosphorus compounds using IPEMA works well for imparting flame retardancy.
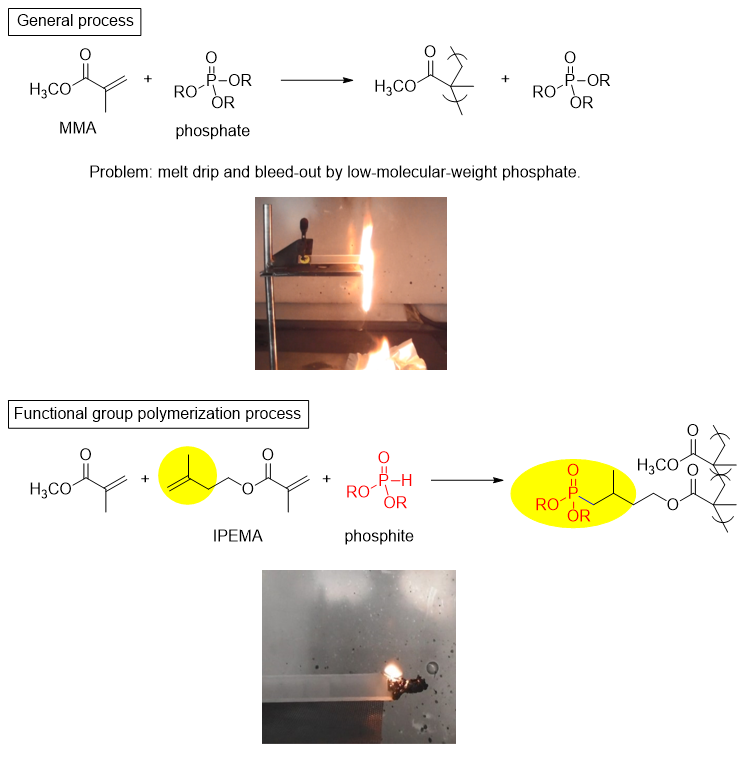 Figure 3. Addition of flame retardancy to poly(methyl methacrylate) using IPEMA
Figure 3. Addition of flame retardancy to poly(methyl methacrylate) using IPEMA
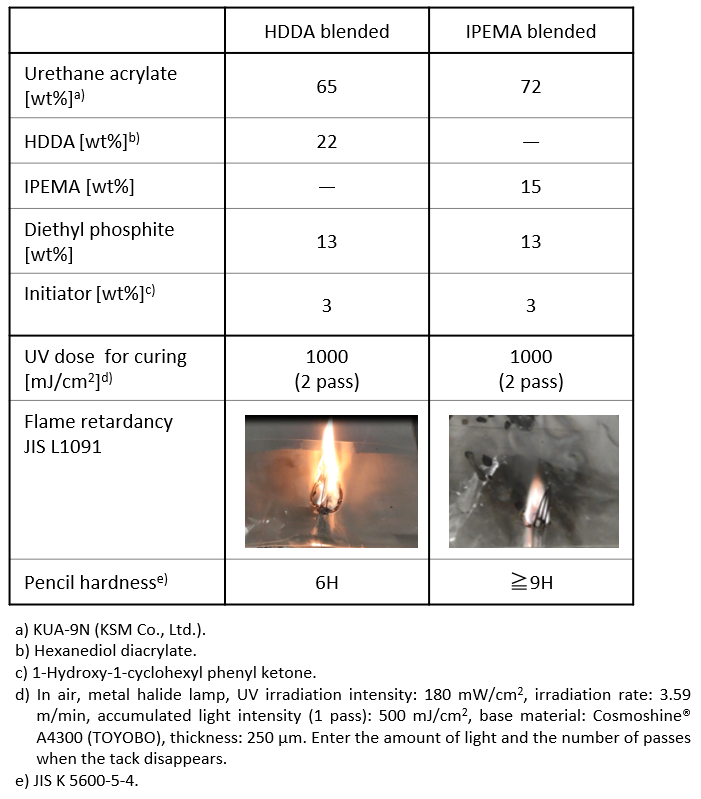
Furthermore, we have applied the phosphorus introduction technology through functionalized polymerization using IPEMA to coatings (Table 3). Compared to the general-purpose bifunctional monomer HDDA, a cured film with excellent flame retardancy and high hardness can be obtained under conditions using IPEMA.
Table 3. Evaluation of flame retardancy in polyurethane cured films
References
1) Grulke, E. A. et al. : “Polymer Handbook”, Wiley, vol. 2 (2003).
* IPEMA is developed by Kuraray Co., Ltd..
Overview / Applications
Property
| Appearance | Colorless - nearly colorless, clear liquid |
|---|
Manufacturer Information
Alias
- Isoprenyl Methacrylate
IPEMA
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.