UV Curing Accelerator
DPNG (Diprenyl Glycerin Ether) and IPEMA (Isoprenyl Methacrylate) are additives that reduce oxygen inhibition and accelerate curing through different mechanisms in UV curing under air. In addition, since DPNG has the function of absorbing and decomposing oxygen by itself, it can impart an antioxidant effect to the resin, and IPEMA is a cross-linking agent with two different radical reactive groups, can be given effects such as flexibility and scratch resistance.
DPNG, IPEMA as a UV Curing Accelerator
UV curing technology uses less solvent and cures faster than heat-based radical polymerization, so it is highly productive, energy-saving, and eco-friendly. A universal problem in UV curing technology is reaction inhibition due to oxygen in the air, and this effect is particularly pronounced for thin films with a large surface area per volume. Common methods to prevent oxygen inhibition include UV curing under a nitrogen purge, attaching a cover to block oxygen, adding oxygen-absorbing compounds such as amines, and increasing the amount of initiator. However, these methods have yet to solve substantial issues due to problems such as increased costs and reduced physical properties of the polymer. New additives DPNG and IPEMA were developed to solve this problem (Figure 1).
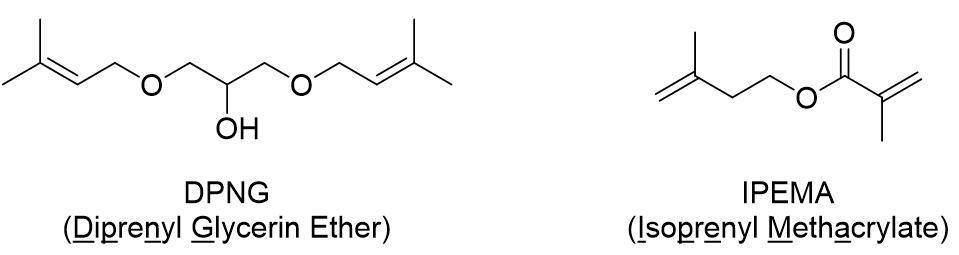
Figure 1. Structure of DPNG and IPEMA
Figure 2 shows the time course of UV curing using DPNG and IPEMA as additives. Pentaerythritol triacrylate was used as a monomer and 1-hydroxycyclohexyl phenyl ketone was used as an initiator, and the change in the conversion of olefin was tracked using real-time IR measurements. While the curing proceeded with the conversion of 74.5% within 30 seconds under oxygen-blocked conditions (example 1), the conversion in the air remained at 4.3% due to the influence of oxygen (example 6). On the other hand, when DPNG or IPEMA was added, the conversion was 46.2% and 44.5%, respectively, even in the air, and the conversion improved by more than 10 times (examples 2 and 3). In addition, looking at the change in the conversion over time, it is also a significant feature that the addition of DPNG or IPEMA causes the reaction rate to rise sharply immediately after UV irradiation, as in the case of the oxygen-blocking condition.
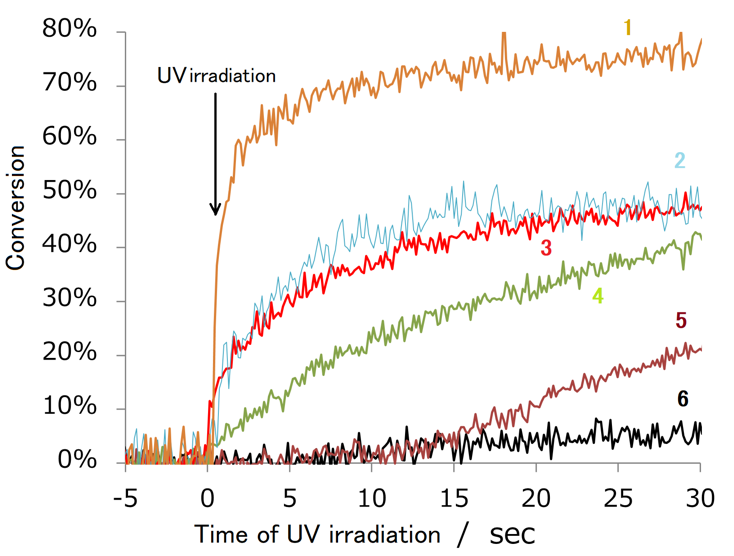
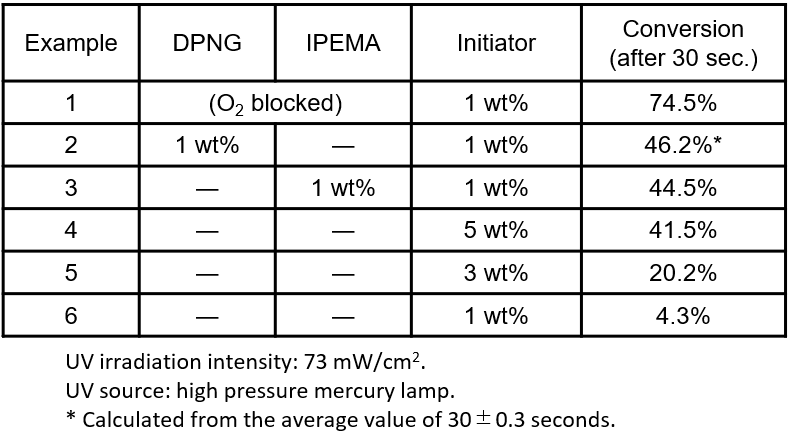
Figure 2. Change over time of olefin conversion by UV irradiation
Figure 3 shows the oxygen absorption mechanism of DPNG estimated from decomposition analysis. During the reaction, a radical is generated at the allylic position of DPNG, which then reacts with oxygen to generate a peroxide radical of DPNG and oxygen is captured. The generated peroxide radicals are thought to regenerate DPNG radicals by abstracting hydrogen from other DPNG, and the oxygen absorption cycle of DPNG proceeds. On the other hand, since IPEMA does not have the ability to absorb oxygen, it is believed that it promotes UV curing through a mechanism different from that of DPNG. Since the addition of isoprenol or methyl methacrylate does not promote UV curing, it is presumed that having an isoprenyl group and a methacryloyl group in the same molecule contributes to the acceleration effect.
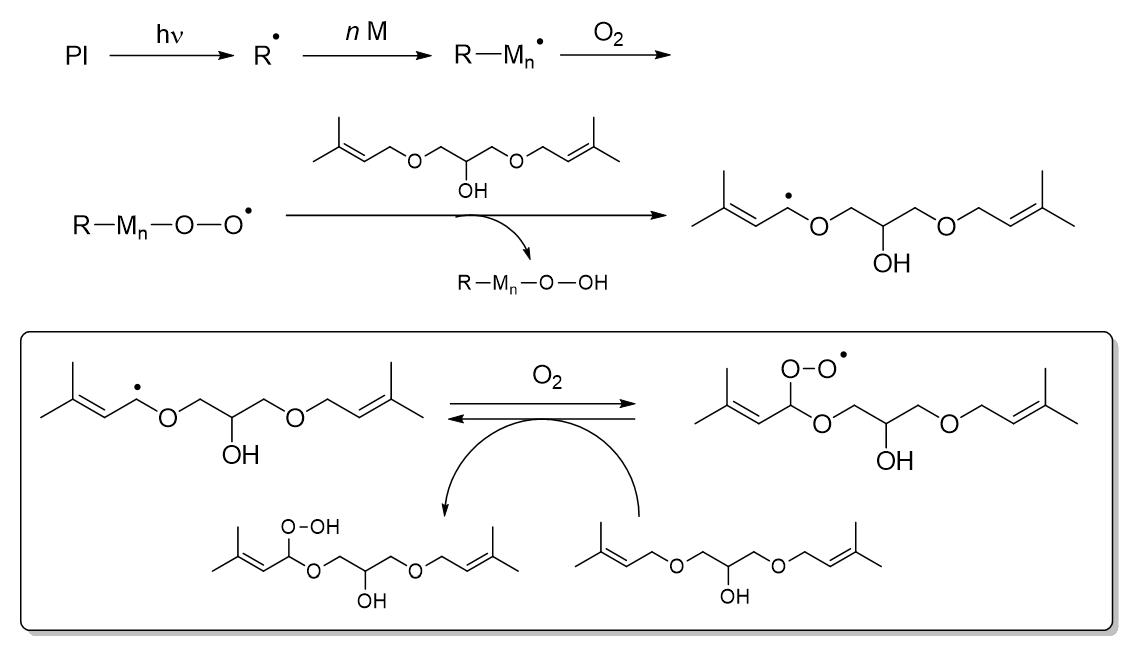
Figure 3. Presumed oxygen absorption mechanism of DPNG
Addition of Functions to Resins - Suppression of Yellowing
Resin components contained in paints and coatings are subject to oxidation over time, and this oxidation causes resin deterioration such as yellowing and cracking. Therefore, antioxidants are added to many paints and coatings. But particularly, phenolic antioxidants can act as a stopping agent for radical polymerization and can cause curing defects, making it difficult in some cases to add a sufficient prescription amount to prevent oxidation.
DPNG has oxygen absorption properties, so it functions as an additive to prevent oxidative deterioration of resins, which is triggered by oxygen. While most common antioxidants are solids, DPNG is a liquid and exhibits high compatibility with resins and coating solution. When DPNG is formulated into a UV-curable composition, DPNG acts as a curing accelerator during UV curing and as an antioxidant to prevent resin deterioration after curing.
Figure 4 shows the weather resistance test of a polyurethane resin containing DPNG. The DPNG-added product has a smaller ΔYI value (a value that indicates the amount of change in the degree of yellowing) compared to non-added products and other antioxidants, indicating that yellowing is suppressed. While existing antioxidants prevent oxidation by quenching radical species and peroxides, DPNG prevents oxidation by absorbing and decomposing oxygen itself. Therefore, a synergistic effect of antioxidant is expected by using DPNG together with existing antioxidants.

Polyurethane resin after weather resistance test
(left) blank, (light) addition of DPNG
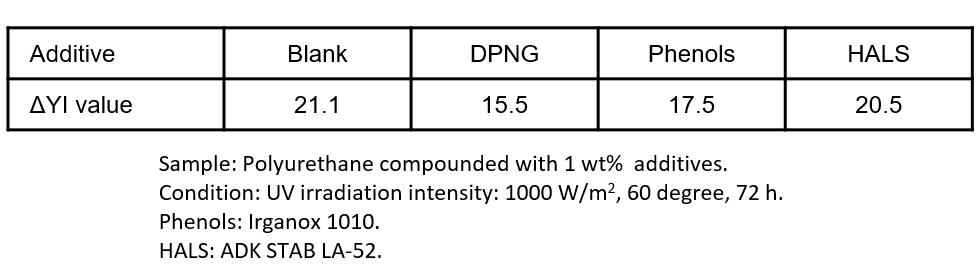
Figure 4. Effects of additives in weather resistance test of polyurethane resins
Addition of Functions to Resins – Both Hardness and Flexibility, Curl Control
Polyfunctional monomers or oligomers added to UV curable paints and coatings have high viscosity, so reactive diluents are added to reduce viscosity and improve handling. Problems associated with the use of reactive diluents include a decrease in curability due to increase the diffusion rate of oxygen as the viscosity of the liquid decreases, and a decrease in the physical properties of the cured film due to a decrease in the density of the functional groups caused by dilution. IPEMA can be used as an effective reactive diluent for these problems.
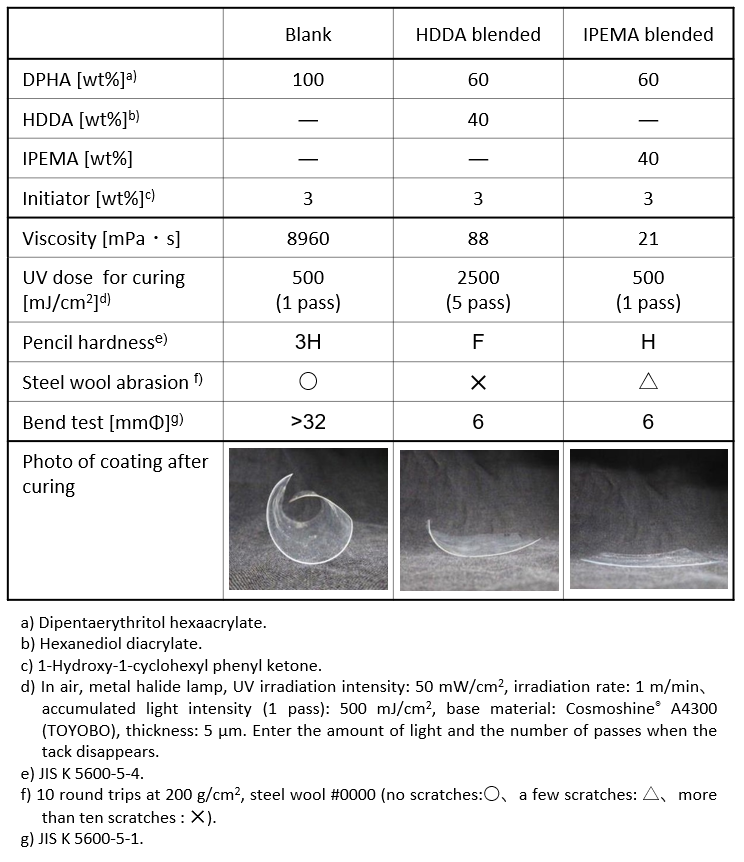
Table 1 shows the physical properties of the cured film when IPEMA is blended with polyfunctional acrylate as a reactive diluent. Compared to the difunctional acrylate monomer HDDA, which is a common reactive diluent, IPEMA has lower viscosity, requires less light for curing, and is less susceptible to oxygen inhibition. Furthermore, the resulting cured film exhibits unique physical properties, including excellent surface hardness and scratch resistance, as well as a high flex resistance of 6 mmΦ in a flex resistance test. It can be said that the cured film containing IPEMA is an interesting material in terms of achieving both hardness and flexibility, which are inherently in a trade-off relationship. In addition, as shown in the photograph, the cured film containing IPEMA shows less warping of the base material, and curling is suppressed compared to the cured film containing HDDA.
These unique physical properties are thought to be derived from the molecular structure of IPEMA, which has a highly reactive methacryloyl group and an isoprenyl group reacted at the end of polymerization in the same molecule. By the difference in the timing of polymerization of the functional groups, it is interpreted that the progress of cross-linking that relieves the stress during curing and the formation of a unique cross-linked structure are proceeding. That is leading to the unique function of IPEMA.
Table 1. Changes in coating properties with reactive diluents
* DPNG and IPEMA are developed by Kuraray Co., Ltd..
Product List
- Open All
- Close All
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



