Zwitterionic Monomers
Zwitterionic monomers are molecules that have a cationic moiety and an anionic moiety in the same molecule. Zwitterionic monomers are mainly known as phosphorylcholine, sulfobetaine, and carboxybetaine, and polymers derived from each monomer are known to exhibit high hydrophilicity and to have a protein nonspecific adsorption control ability comparable to that of PEG. Therefore, these polymers are applied to medical devices such as stents and catheters, hydrogels, and drug delivery systems.
About Zwitterionic Polymers
Biocompatible polymers are essential for medical devices used in direct contact with the living body and bio-related devices for cell and tissue culture from the viewpoints of safety, stability, and not affecting biological components, and are still being actively studied1). Water-soluble polymers are mainly used as biocompatible polymers, and polyvinylpyrrolidone, polyoxazoline, poly(N-acryloylmorpholine), and polyethylene glycol (PEG) are known. Common properties of these water-soluble polymers are that they are nonionic and hydrophilic. It is important for biocompatible polymers to inhibit nonspecific adsorption of proteins, and they must not have physical properties with strong interactions (hydrophobicity, charge, dipolarity). On the other hand, these are water soluble, so hydrogen bonding is required. Whitesides reported that molecules with hydrogen bonding acceptors are less prone to nonspecific adsorption than those with donors2). PEG has only oxygen atom of hydrogen-bonding donor as its hydrophilic part and low hydrophobicity, which means that its chemical structure also makes it difficult for protein to adsorb. In addition, compared to proteins and nucleic acids, which form higher-order structures, water-soluble polymers are more bendable without forming structures, which also contributes to the fact that recognition and adsorption by proteins are less likely to occur. Particularly, PEG is known to have the highest flexibility among all water-soluble polymers because of the ether bond in its main chain.
Zwitterionic polymers having cationic and anionic moieties in the same molecule, are known to have protein nonspecific adsorption inhibitory ability comparable to that of PEG. Sulfobetaine, carboxybetaine, phosphorylcholine, dimethylamine oxide, and dimethylsulfoniopropionate have been reported as zwitterions constituting polymers (Table 1). Zwitterions are also abundant in vivo, for example, phosphorylcholine is employed as the head group of phospholipids, and carboxybetaine is present in cells as an osmotic regulator (osmolytes).
| Building Blocks | Structure |
|---|---|
| Sulfobetaine |  |
| Carboxybetaine |  |
| Phosphorylcholine (Phosphobetaine) |
 |
| Dimethylamine oxide (DMAO) |
 |
| Dimethylsulfoniopropionate |  |
A typical example of a zwitterionic polymer is a polymer with 2-methacryloyloxyethyl phosphorylcholine (MPC) as one component (Figure 1).3) Production of MPC polymers began in industrial plants in 1999. The purity of MPC polymers is currently very high as a medical grade, and the safety of the resulting MPC polymers is assured. The purity of MPC polymer is extremely high as a medical grade, and the safety of the MPC obtained is assured and registered in the Master Access File of the U.S. Food and Drug Administration (FDA). MPC polymers have been used for surface treatment of contact lenses, catheters, artificial lungs (ECMO), intravascular stents, etc., and have been in clinical use for more than 25 years. In Japan, MPC polymers have been used as a surface treatment material for the EVAHEART® implantable artificial heart, for which clinical trials began in 2005 and 2011, respectively.
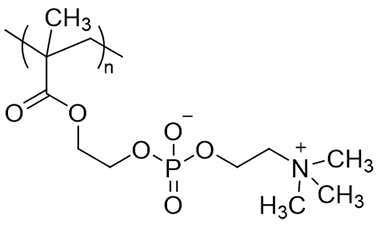
Figure 1. Structure of MPC polymer
A zwitterion are neutralized by intra- and intermolecular ion-pair interactions, making them less likely to some interaction with proteins. In the case of biogenic zwitterions, carboxylic or phosphoric acid is used as the anionic part and quaternary ammonium as the cationic part. Quaternary ammonium has a positively charged nitrogen atom covered with an alkyl group, which prevents the formation of strong hydrogen bonds. This is thought to be effective in inhibiting nonspecific adsorption of proteins. The spacer length between the anionic and cationic parts of the zwitterion is also important in inhibiting protein adsorption. Jiang et al. investigated protein adsorption on polycarboxybetaine (PCB) polymers (Figure 2) with different spacer lengths and found that protein adsorption was more strongly inhibited when the spacer carbon number (m) was smaller than two4).
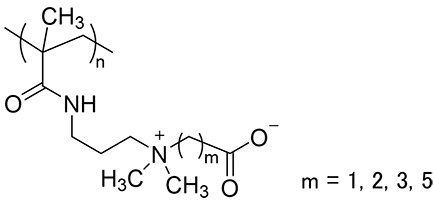
Figure 2. Structure of PCB polymer
References
- Erfani, A., Seaberg, J., Aichele, C. P., Ramsey, J. D. : Biomacromolecules, 21, 2557 (2020).
- Ostuni, E., Chapman, R. G., Holmlin, R. E., Takayama, S., Whitesides, G. M. : Langmuir, 17 (18), 5605 (2001).
- Ishihara, K. : Langmuir, 35 (5), 1778 (2019).
- Zhang, Z., Vaisocherová, G., Cheng, G., Yang, W., Xue, H., Jiang, S. : Biomacromolecules, 9, 2686 (2008).
Product List
- Open All
- Close All
Phosphorylcholine monomer
Sulfobetaine monomer
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



